| CHD1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | CHD1, chromodomain helicase DNA binding protein 1, PILBOS, CHD-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 602118 MGI: 88393 HomoloGene: 68174 GeneCards: CHD1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
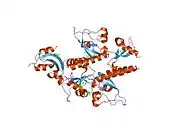
The Chromodomain-Helicase DNA-binding 1 is a protein that, in humans, is encoded by the CHD1 gene.[5][6][7] CHD1 is a chromatin remodeling protein that is widely conserved across many eukaryotic organisms, from yeast to humans. CHD1 is named for three of its protein domains: two tandem chromodomains, its ATPase catalytic domain, and its DNA-binding domain (Figure 1).[8][9]
The CHD1 remodeler binds nucleosomes and induces local changes in nucleosome positioning through ATP hydrolysis coupled to DNA translocation of the DNA across the histone proteins.[8] The catalytic domain of CHD1, which is highly conserved across all nucleosome remodelers, is a two-lobed structure.[8] CHD1 relies on the DNA-binding domain, which binds DNA in a sequence non-specific manner, to help regulate spacing.[10]
CHD1 is a member of a large family of CHD nucleosome remodelers, though yeast has only one CHD protein, called Chd1.[11] Humans and mice, by contrast, have ten CHD proteins that are homologous to CHD1, but each have their own characteristic functions.[11][12]
Structure
CHD1 contains two tandem N-terminal chromodomains, a SNF2-related domain, a helicase C domain, CDH1/2 SANT-Helical linker, and a disordered C-terminal region.[13]
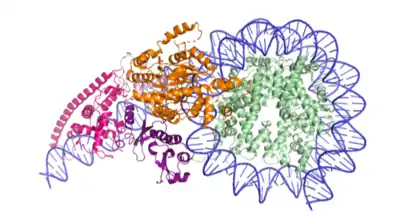
The structure of Chd1 bound to the nucleosome has been solved (Figure 2).[9]
Function
CHD1 is essential for embryonic stem cell pluripotency in mice by maintaining an open euchromatic chromatin state.[14] Chd1 helps maintain boundaries between histone modifications H3K4me3 and H3K36me3.[15] It has also been shown that CHD1 is important in dictating the transcriptional landscape by promoting differentiation of osteoblasts, or differentiating bone cells.[16] Studies in both yeast and humans have found that Chd1 is recruited to DNA damage sites, where it promotes the opening of chromatin and the recruitment of DNA repair factors, thus facilitating DNA repair by homologous recombination. [17][18]
Interactions
CHD1 has several genetic interactions with numerous factors involved in chromatin maintenance and transcription. Notably, the chromodomains of human CHD1 are capable of binding the histone modification histone H3 Lysine 4 trimethyl (H3K4me3).[19] It is thought that human CHD1 preferentially binds this histone modification, which is primarily located at the 5’ regions of genes, as a mechanism of recruitment to those genomic loci. However, in yeast it has been shown that Chd1 interacts with Rtf1, a transcription elongation factor and member of the Paf1 Complex (Paf1C).[20] Structural information has shown that the Chd1 chromodomains in yeast do not bind H3K4me3.[11]
CHD1 has been shown to interact with Nuclear receptor co-repressor 1.[21]
Clinical significance
CHD1 is most notably implicated in prostate cancer development. In about 10% of all prostate cancers, CHD1 is mutated or deleted.[22][23] In prostate cancer cells CHD1 also has an essential relationship with another cancer driver, the PTEN locus. In studies of prostate cancer patient data, when PTEN is mutated, Chd1 gains an essential role and is never deleted.[22] Thus, CHD1 misfunction is evident in the majority of prostate cancers. Further, mutation of CHD1 alone is sufficient in some mice models to induce prostate tumorigenesis.[24]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000153922 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000023852 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ Delmas V, Stokes DG, Perry RP (March 1993). "A mammalian DNA-binding protein that contains a chromodomain and an SNF2/SWI2-like helicase domain". Proceedings of the National Academy of Sciences of the United States of America. 90 (6): 2414–8. Bibcode:1993PNAS...90.2414D. doi:10.1073/pnas.90.6.2414. PMC 46097. PMID 8460153.
- ↑ Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS (October 1997). "Characterization of the CHD family of proteins". Proceedings of the National Academy of Sciences of the United States of America. 94 (21): 11472–7. Bibcode:1997PNAS...9411472W. doi:10.1073/pnas.94.21.11472. PMC 23509. PMID 9326634.
- ↑ "Entrez Gene: CHD1 chromodomain helicase DNA binding protein 1".
- 1 2 3 Clapier CR, Iwasa J, Cairns BR, Peterson CL (July 2017). "Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes". Nature Reviews. Molecular Cell Biology. 18 (7): 407–422. doi:10.1038/nrm.2017.26. PMC 8127953. PMID 28512350.
- 1 2 Farnung L, Vos SM, Wigge C, Cramer P (October 2017). "Nucleosome-Chd1 structure and implications for chromatin remodelling". Nature. 550 (7677): 539–542. Bibcode:2017Natur.550..539F. doi:10.1038/nature24046. PMC 5697743. PMID 29019976.
- ↑ Hauk G, McKnight JN, Nodelman IM, Bowman GD (September 2010). "The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor". Molecular Cell. 39 (5): 711–23. doi:10.1016/j.molcel.2010.08.012. PMC 2950701. PMID 20832723.
- 1 2 3 Flanagan JF, Blus BJ, Kim D, Clines KL, Rastinejad F, Khorasanizadeh S (June 2007). "Molecular implications of evolutionary differences in CHD double chromodomains". Journal of Molecular Biology. 369 (2): 334–42. doi:10.1016/j.jmb.2007.03.024. PMC 1948097. PMID 17433364.
- ↑ Cheng W, Su Y, Xu F (December 2013). "CHD1L: a novel oncogene". Molecular Cancer. 12 (1): 170. doi:10.1186/1476-4598-12-170. PMC 3931672. PMID 24359616.
- ↑ "CHD1_HUMAN (O14646)". InterPro. European Molecular Biology Laboratory.
- ↑ Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, et al. (August 2009). "Chd1 regulates open chromatin and pluripotency of embryonic stem cells". Nature. 460 (7257): 863–8. Bibcode:2009Natur.460..863G. doi:10.1038/nature08212. PMC 3891576. PMID 19587682.
- ↑ Lee Y, Park D, Iyer VR (July 2017). "The ATP-dependent chromatin remodeler Chd1 is recruited by transcription elongation factors and maintains H3K4me3/H3K36me3 domains at actively transcribed and spliced genes". Nucleic Acids Research. 45 (12): 7180–7190. doi:10.1093/nar/gkx321. PMC 5499586. PMID 28460001.
- ↑ Baumgart SJ, Najafova Z, Hossan T, Xie W, Nagarajan S, Kari V, et al. (July 2017). "CHD1 regulates cell fate determination by activation of differentiation-induced genes". Nucleic Acids Research. 45 (13): 7722–7735. doi:10.1093/nar/gkx377. PMC 5570082. PMID 28475736.
- ↑ Gnugnoli, M; Casari, E; Longhese, MP (September 2021). "The chromatin remodeler Chd1 supports MRX and Exo1 functions in resection of DNA double-strand breaks". PLOS Genetics. 17 (9): e1009807. doi:10.1371/journal.pgen.1009807. PMC 8462745. PMID 34520455.
- ↑ Kari, V; Mansour, WY; Raul, SK; Baumgart, SJ; Mund, A; Grade, M; Sirma, H; Simon, R; Will, H; Dobbelstein, M; Dikomey, E; Johnsen, SA (November 2016). "Loss of CHD1 causes DNA repair defects and enhances prostate cancer therapeutic responsiveness". EMBO Reports. 17 (11): 1609–1623. doi:10.15252/embr.201642352. PMC 5090703. PMID 27596623.
- ↑ Flanagan JF, Mi LZ, Chruszcz M, Cymborowski M, Clines KL, Kim Y, et al. (December 2005). "Double chromodomains cooperate to recognize the methylated histone H3 tail". Nature. 438 (7071): 1181–5. Bibcode:2005Natur.438.1181F. doi:10.1038/nature04290. PMID 16372014. S2CID 4401500.
- ↑ Simic R, Lindstrom DL, Tran HG, Roinick KL, Costa PJ, Johnson AD, et al. (April 2003). "Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes". The EMBO Journal. 22 (8): 1846–56. doi:10.1093/emboj/cdg179. PMC 154471. PMID 12682017.
- ↑ Tai HH, Geisterfer M, Bell JC, Moniwa M, Davie JR, Boucher L, McBurney MW (August 2003). "CHD1 associates with NCoR and histone deacetylase as well as with RNA splicing proteins". Biochemical and Biophysical Research Communications. 308 (1): 170–6. doi:10.1016/S0006-291X(03)01354-8. PMID 12890497.
- 1 2 Zhao D, Lu X, Wang G, Lan Z, Liao W, Li J, et al. (February 2017). "Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer". Nature. 542 (7642): 484–488. Bibcode:2017Natur.542..484Z. doi:10.1038/nature21357. PMC 5448706. PMID 28166537.
- ↑ Huang S, Gulzar ZG, Salari K, Lapointe J, Brooks JD, Pollack JR (September 2012). "Recurrent deletion of CHD1 in prostate cancer with relevance to cell invasiveness". Oncogene. 31 (37): 4164–70. doi:10.1038/onc.2011.590. PMC 5512870. PMID 22179824.
- ↑ Augello MA, Liu D, Deonarine LD, Robinson BD, Huang D, Stelloo S, et al. (April 2019). "CHD1 Loss Alters AR Binding at Lineage-Specific Enhancers and Modulates Distinct Transcriptional Programs to Drive Prostate Tumorigenesis". Cancer Cell. 35 (4): 603–617.e8. doi:10.1016/j.ccell.2019.03.001. PMC 6467783. PMID 30930119.
Further reading
- Stokes DG, Perry RP (May 1995). "DNA-binding and chromatin localization properties of CHD1". Molecular and Cellular Biology. 15 (5): 2745–53. doi:10.1128/mcb.15.5.2745. PMC 230505. PMID 7739555.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Kelley DE, Stokes DG, Perry RP (April 1999). "CHD1 interacts with SSRP1 and depends on both its chromodomain and its ATPase/helicase-like domain for proper association with chromatin". Chromosoma. 108 (1): 10–25. doi:10.1007/s004120050347. PMID 10199952. S2CID 12945778.
- Salomon AR, Ficarro SB, Brill LM, Brinker A, Phung QT, Ericson C, et al. (January 2003). "Profiling of tyrosine phosphorylation pathways in human cells using mass spectrometry". Proceedings of the National Academy of Sciences of the United States of America. 100 (2): 443–8. Bibcode:2003PNAS..100..443S. doi:10.1073/pnas.2436191100. PMC 141014. PMID 12522270.
- Tai HH, Geisterfer M, Bell JC, Moniwa M, Davie JR, Boucher L, McBurney MW (August 2003). "CHD1 associates with NCoR and histone deacetylase as well as with RNA splicing proteins". Biochemical and Biophysical Research Communications. 308 (1): 170–6. doi:10.1016/S0006-291X(03)01354-8. PMID 12890497.
- Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, et al. (June 2004). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation". Nature Biotechnology. 22 (6): 707–16. doi:10.1038/nbt971. PMID 15146197. S2CID 27764390.
- Sims RJ, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D (December 2005). "Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains". The Journal of Biological Chemistry. 280 (51): 41789–92. doi:10.1074/jbc.C500395200. PMC 1421377. PMID 16263726.
- Flanagan JF, Mi LZ, Chruszcz M, Cymborowski M, Clines KL, Kim Y, et al. (December 2005). "Double chromodomains cooperate to recognize the methylated histone H3 tail". Nature. 438 (7071): 1181–5. Bibcode:2005Natur.438.1181F. doi:10.1038/nature04290. PMID 16372014. S2CID 4401500.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983. S2CID 7827573.
- Okuda M, Horikoshi M, Nishimura Y (January 2007). "Structural polymorphism of chromodomains in Chd1". Journal of Molecular Biology. 365 (4): 1047–62. doi:10.1016/j.jmb.2006.10.039. PMID 17098252.
- Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
External links
- Human CHD1 genome location and CHD1 gene details page in the UCSC Genome Browser.