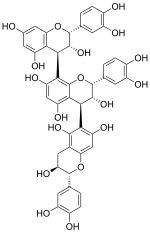
 | |
| Names | |
|---|---|
| IUPAC name
[(2R,3R,4R)-Flavan-3,3′,4′,5,7-pentol]-(4→8)-[(2R,3R,4S)-flavan-3,3′,4′,5,7-pentol]-(4→6)-[(2R,3S)-flavan-3,3′,4′,5,7-pentol] | |
| Systematic IUPAC name
(12R,13R,14R,22R,23R,24S,32R,33S)-12,22,32-Tris(3,4-dihydroxyphenyl)-13,14,23,24,33,34-hexahydro-12H,22H,32H-[14,28:24,36-ter-1-benzopyran]-13,15,17,23,25,27,33,35,37-nonol | |
| Other names
Epicatechin-(4β→8)-epicatechin-(4β→6)-catechin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C45H38O18 | |
| Molar mass | 866.77 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Arecatannin B1 is a B type proanthocyanidin found in the betel nut.[1] It is an arecatannin trimer with a 4β→6 bond.
References
- ↑ Screening of various plant extracts used in Ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Kusumoto I.T., Nakabayashi T., Kida H., Miyashiro H., Hattori M., Namba T. and Shimotohno K., PTR. Phytotherapy research, 1995, vol. 9, no3, pp. 180-184, INIST 3499215
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.