| 5-formyltetrahydrofolate cyclo-ligase | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | methenyl-THF synthetase5,10-methenyltetrahydrofolate synthetase5-formyltetrahydrofolate cyclo-ligase (ADP-forming)5-Formyltetrahydrofolate cyclodehydraseformyltetrahydrofolic cyclodehydrase | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 604197 GeneCards: | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-Formyltetrahydrofolate cyclo-ligase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 6.3.3.2 | ||||||||
| CAS no. | 37318-64-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
In enzymology, a 5-formyltetrahydrofolate cyclo-ligase (EC 6.3.3.2) is an enzyme that catalyzes the chemical reaction
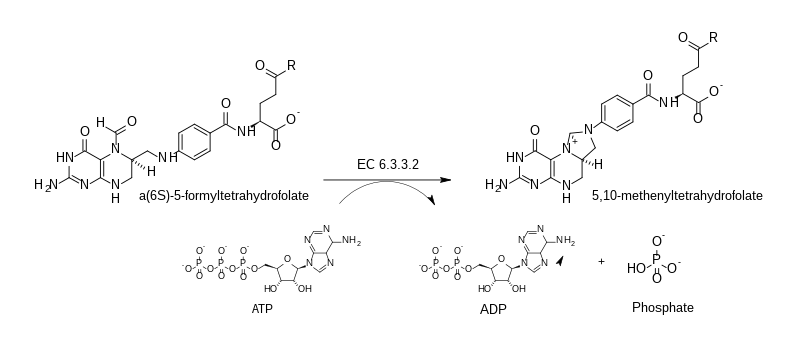
- ATP + 5-formyltetrahydrofolate (folinic acid) ADP + phosphate + 5,10-methenyltetrahydrofolate
Thus, the two substrates of this enzyme are ATP and 5-formyltetrahydrofolate, whereas its 3 products are ADP, phosphate, and 5,10-methenyltetrahydrofolate.
This enzyme belongs to the family of ligases, specifically the cyclo-ligases, which form carbon-nitrogen bonds. The systematic name of this enzyme class is 5-formyltetrahydrofolate cyclo-ligase (ADP-forming). Other names in common use include 5,10-methenyltetrahydrofolate synthetase (MTHFS), formyltetrahydrofolic cyclodehydrase, and 5-formyltetrahydrofolate cyclodehydrase. This enzyme participates in one carbon pool by folate.
Structural studies
As of late 2007, 5 structures have been solved for this class of enzymes, with PDB accession codes 1SBQ, 1SOU, 1U3F, 1U3G, and 2JCB.
Role in pathology
Mutations of the MTHFS gene cause the disease 5,10-methenyltetrahydrofolate synthetase deficiency.
References
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Greenberg DM, Wynston LK, Nagabhushanan A (1965). "Further studies on N5-formyltetrahydrofolic acid cyclodehydrase". Biochemistry. 4 (9): 1872–1878. doi:10.1021/bi00885a026.