 | |
| Names | |
|---|---|
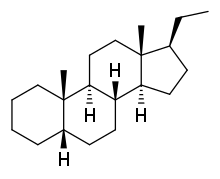
| IUPAC name
5β-Pregnane | |
| Systematic IUPAC name
(1S,3aS,3bS,5aS,9aS,9bS,11aR)-1-Ethyl-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Other names
17β-Ethyletiocholane; 17β-Ethyl-5β-androstane; 10β,13β-Dimethyl-17β-ethyl-5β-gonane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.905 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H36 | |
| Molar mass | 288.519 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5β-Pregnane, also known as 17β-ethyletiocholane or as 10β,13β-dimethyl-17β-ethyl-5β-gonane, is a steroid and a parent compound of a variety of steroid derivatives.[1] It is one of the epimers of pregnane, the other being 5α-pregnane. Derivatives of 5β-pregnane include the naturally occurring steroids 5β-dihydroprogesterone, pregnanolone, epipregnanolone, pregnanediol, and pregnanetriol, and the synthetic steroids hydroxydione, renanolone, ORG-20599, and SAGE-217. These derivatives include metabolites of progesterone and endogenous and synthetic neurosteroids.
See also
References
- ↑ William M. Haynes (22 June 2016). CRC Handbook of Chemistry and Physics, 97th Edition. CRC Press. pp. 3–. ISBN 978-1-4987-5429-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.