 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 1-2 hrs |
| Identifiers | |
| |
| CAS Number |
|
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.214.853 |
| Chemical and physical data | |
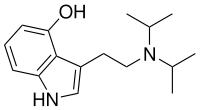
| Formula | C16H24N2O |
| Molar mass | 260.381 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

4-Hydroxy-N,N-diisopropyltryptamine (4-HO-DiPT or Iprocin) is a synthetic psychedelic drug. It is a higher homologue of psilocin, 4-HO-DET, and is a positional isomer of 4-HO-DPT and has a tryptamine molecular sub-structure.
Dosage
4-HO-DiPT is orally active at around 3 mg and above, and its effects last for 2–3 hours.[1] Higher doses such as those above 30 mgs can increase the duration of the effects significantly.
Effects
The effects of 4-HO-DiPT are broadly comparable to those of other serotonergic psychedelics such as LSD and psilocin, but they are distinguished by their relative brevity. Shulgin "doubt[s] that there is another psychedelic drug, anywhere, that can match this one for speed, for intensity, for brevity, and sensitivity to dose, at least one that is active orally." An idiosyncratic effect of the drug, also noted by Shulgin, is its tendency to induce tremors.[2][3][4]
Some users have reported a minor audio distortion with lower dosages. Higher dosages increase the polarity of the distortion. It is defined as being slightly lower in pitch and creating several different effects, such as pitch bend, volume distortion, and rate distortion. As with most DiPT psychedelics, music can become more dissonant and less harmonious. Users have also reported a visual distortion widely comparable to the hallucinogen LSD.
Pharmacology
Pharmacodynamics
| Binding Sites | Binding Affinity Ki (μM) | Action [5] |
|---|---|---|
| 5-HT1A | 5.7 | ??? |
| 5-HT2A | 0.728 | partial agonist |
| 5-HT2C | 2.8 | ??? |
| D1 | >25 | ??? |
| D2 | >25 | ??? |
| D3 | >25 | ??? |
| α1A | >12 | ??? |
| α2A | 15 | ??? |
| TAAR1 | >15 | ??? |
| H1 | 9.8 | ??? |
| SERT | 1.8 | inhibitor |
| DAT | >26 | inhibitor |
| NET | 11 | inhibitor |
Clinical trials
FT-104, a prodrug to 4-HO-DiPT, has entered double blind, randomized, placebo controlled, phase 1 clinical trials in healthy volunteers at the Royal Adelaide Hospital in Australia for the treatment of postpartum depression and treatment-resistant depression. [6][7][8] It is propably a less neurotoxic alternative to alpha-Methyltryptamine (αMT, AMT, Indopan) and alpha-Ethyltryptamine (αET, AET, Monase).
Legal status
Finland
Scheduled in government decree on psychoactive substances banned from the consumer market.[9]
Germany
Scheduled in New Psychoactive Substances Act (NpSG). Use of covered substances is permitted only for industrial and scientific purposes.
Sweden
Sveriges riksdags health ministry Statens folkhälsoinstitut classified 4-HO-DiPT as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Mar 1, 2005, in their regulation SFS 2005:26 listed as 4-hydroxi-N,N-diisopropyltryptamin (4-HO-DIPT), making it illegal to sell or possess.[10]
United States
4-HO-DiPT is not scheduled at the federal level in the United States,[11] but it is possible that it could be considered an analog of 5-MeO-DiPT, in which case purchase, sale, or possession could be prosecuted under the Federal Analog Act.
Florida
"4-Hydroxy-N,N-diisopropyltryptamine" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[12]
References
- ↑ "Erowid 4-HO-DiPT Vault : Dosage". www.erowid.org. Retrieved 8 April 2023.
- ↑ Shulgin, Alexander; Shulgin, Ann (September 1997). TiHKAL: The Continuation. Berkeley, California: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252.
- ↑ "4-Hydroxy-DIPT". Erowid.
- ↑ "Tihkal 4-HO-DIPT entry".
- ↑ Rickli A, Moning OD, Hoener MC, Liechti ME (August 2016). "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens" (PDF). European Neuropsychopharmacology. 26 (8): 1327–37. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. S2CID 6685927.
- ↑ "Field Trip Announces First Dosings in Phase I Clinical Study of FT-104" (Press release). 21 July 2022.
- ↑ "An Inside Look into Field Trip's Next-Generation Psychedelic, FT-104". 11 August 2022.
- ↑ "WIPO - Search International and National Patent Collections". patentscope.wipo.int. Retrieved 8 April 2023.
- ↑ "FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 1130/2014". www.finlex.fi. Retrieved 8 April 2023.
- ↑ "Svensk författningssamling" [Swedish Code of Statutes] (PDF) (in Swedish). Archived from the original (PDF) on 29 September 2013. Retrieved 25 September 2013.
- ↑ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I." Archived from the original on 27 August 2009. Retrieved 17 December 2014.
- ↑ "Statutes & Constitution :View Statutes : Online Sunshine". leg.state.fl.us. Retrieved 8 April 2023.
