 | |
| Names | |
|---|---|
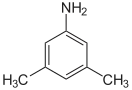
| Preferred IUPAC name
3,5-Dimethylaniline | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.280 |
| EC Number |
|
| MeSH | C514328 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 0077 1711 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H11N | |
| Molar mass | 121.183 g·mol−1 |
| Appearance | colorless oil |
| Density | 0.9704 g/cm3 |
| Melting point | 9.8–10.0 °C (49.6–50.0 °F; 282.9–283.1 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Hazards | |
| GHS labelling: | |
 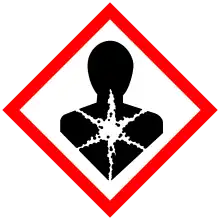 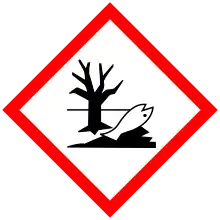 | |
| Danger | |
| H301, H311, H331, H373, H411 | |
| P260, P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P311, P312, P314, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| Flash point | 103 °C (217 °F; 376 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3,5-Xylidine is the organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. It is used in the production of the dye Pigment Red 149.[1]

Chemical structure of Pigment Red 149
Production
3,5-Xylidine is produced industrially by amination of the xylenol using ammonia and alumina catalyst.
References
- ↑ M. Meyer (2012). "Xylidines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_455. ISBN 978-3527306732.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.