 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,5-Dimethylaniline | |
| Other names
2,5-Dimethylphenylamine, 2,5-Dimethylbenzenamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.229 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H11N | |
| Melting point | 6 °C (43 °F; 279 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Hazards | |
| Flash point | 97 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,5-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives include Solvent Yellow 30, Solvent Red 22, Acid Red 65, and Solvent Red 26.[1]
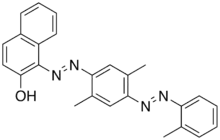 Solvent Red 26 is a commercial dye prepared from 2,5-xylidine
Solvent Red 26 is a commercial dye prepared from 2,5-xylidine
Production
Like many xylidines, it is prepared by nitration of the corresponding xylene followed by reduction of the nitroxylene. Reduction can be effected with HCl/Fe, but usually is achieved by catalytic hydrogenation:
- Me2C6H4 + HNO3 → Me2C6H3NO2 + H2O
- Me2C6H3NO2 + 3 H2 → Me2C6H3NH2 + 3H2O
Safety
It is mutagenic and tumor-inducing. Acute toxicity of xylidines is modest as indicated by LD50 (rats, oral) are in the range 0.1-1 g/kg.
References
- ↑ M. Meyer (2012). "Xylidines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_455. ISBN 9783527303854.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.