 | |
| Names | |
|---|---|
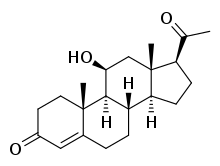
| IUPAC name
11β-Hydroxypregn-4-ene-3,20-dione | |
| Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,10S,11aS)-1-Acetyl-10-hydroxy-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
11β-OHP; 21-Deoxycorticosterone; 21-Desoxycorticosterone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.088 |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H30O3 | |
| Molar mass | 330.468 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
11β-Hydroxyprogesterone (11β-OHP), also known as 21-deoxycorticosterone, as well as 11β-hydroxypregn-4-ene-3,20-dione, is a naturally occurring, endogenous steroid and derivative of progesterone.[1] It is a potent mineralocorticoid.[1] Syntheses of 11β-OHP from progesterone is catalyzed by the steroid 11β-hydroxylase (CYP11B1) enzyme,[2][3] and, to a lesser extent, by the aldosterone synthase enzyme (CYP11B2).[2]
Function
Along with its epimer 11α-hydroxyprogesterone (11α-OHP), 11β-OHP has been identified as a very potent competitive inhibitor of both isoforms (1 and 2) of 11β-hydroxysteroid dehydrogenase (11β-HSD).[4][5]
Outcome of 21-hydroxylase deficiency
It has been known since 1987 that increased levels of 11β-OHP occur in 21-hydroxylase deficiency.[6][7] A study in 2017 has shown that in subjects with 21-hydroxylase deficiency, serum 11β-OHP concentrations range from 0.012 to 3.37 ng/mL, while in control group it was below detection limit of 0.012 ng/mL.[8] 21-hydroxylase is an enzyme that is also involved in progesterone metabolism, producing 11-deoxycorticosterone. In normal conditions, 21-hydroxylase has higher activity on progesterone than steroid 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) that convert progesterone to 11β-OHP. That's why in 21-hydroxylase deficiency, given the normal function of the CYP11B enzymes, the progesterone is directed towards 11β-OHP pathway rather than towards 11-deoxycorticosterone pathway, that is also usually accompanied by an increase in progesterone levels.[9] In the normal route to aldosterone and cortisol, progesterone and 17α-hydroxyprogesterone are first hydroxylated at position 21 and then hydroxylated at other positions. In 21-hydroxylase deficiency, progesterone and 17α-hydroxyprogesterone accumulate and are the substrates of steroid 11β-hydroxylase, leading to 1β-OHP and 21-deoxycortisol, respectively.[10] In the 2017 study mentioned above, serum progesterone concentrations in boys (10 days to 18 years old) with 21-hydroxylase deficiency reached levels similar to female luteal values (up to 10.14 ng/mL, depending on severity and treatment), while in the control group of boys progesterone was 0.07 ng/mL (0.22 nmol/L) on average, ranged from 0.05 to 0.40 ng/mL.[8]
In a 2016 study, classical CAH patients receiving glucocorticoid therapy had C19 11-oxygenated steroid serum levels that were elevated 3-4 fold compared to healthy controls.[11] In that same study, the levels of C19 11-oxygenated androgens correlated positively with conventional androgens in women but negatively in men. The levels of 11KT were four times higher than that of T in women with the condition. In adult women with CAH, the ratio of DHT produced in a backdoor pathway to that produced in a conventional pathway increases as control of androgen excess by glucocorticoid therapy deteriorates.[12] In CAH patients with poor disease control, 11-oxygenated androgens remain elevated for longer than 17OHP, thus serving as a better biomarker for the effectiveness of the disease control.[13][14] In males with CAH, 11-oxygenated androgen levels may indicate the presence testicular adrenal rest tumors.[14][15][16]
While studies suggest that 11β-OHP, also known as 21-deoxycorticosterone, can be used as marker for adrenal 21-hydroxylase deficiency,[6] another 21-carbon steroid — 21-deoxycortisol (produced from 17α-hydroxyprogesterone) gained acceptance for this purpose.[17][18][19]
See also
- 21-Deoxycortisol (11β,17α-dihydroxyprogesterone)
- 11-Deoxycorticosterone (21-hydroxyprogesterone)
- Corticosterone (11β,21-dihydroxyprogesterone)
- Cortisol (11β,17α,21-trihydroxyprogesterone)
- 11-Deoxycortisol (17α,21-dihydroxyprogesterone)
- 9α-Bromo-11-ketoprogesterone
References
 This article incorporates text available under the CC BY-SA 3.0 license.
This article incorporates text available under the CC BY-SA 3.0 license.
- 1 2 "Human Metabolome Database: Showing metabocard for 11b-Hydroxyprogesterone (HMDB04031)". hmdb.ca. Retrieved 16 December 2016.
- 1 2 Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, Park HW (February 2013). "Structural insights into aldosterone synthase substrate specificity and targeted inhibition". Molecular Endocrinology. 27 (2): 315–24. doi:10.1210/me.2012-1287. PMC 5417327. PMID 23322723.
- ↑ van Rooyen D, Gent R, Barnard L, Swart AC (April 2018). "The in vitro metabolism of 11β-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology. 178: 203–212. doi:10.1016/j.jsbmb.2017.12.014. PMID 29277707. S2CID 3700135.
- ↑ Souness GW, Latif SA, Laurenzo JL, Morris DJ (April 1995). "11 alpha- and 11 beta-hydroxyprogesterone, potent inhibitors of 11 beta-hydroxysteroid dehydrogenase (isoforms 1 and 2), confer marked mineralocorticoid activity on corticosterone in the ADX rat". Endocrinology. 136 (4): 1809–12. doi:10.1210/endo.136.4.7895695. PMID 7895695.
- ↑ Souness GW, Morris DJ (March 1996). "11 alpha- and 11 beta-hydroxyprogesterone, potent inhibitors of 11 beta-hydroxysteroid dehydrogenase, possess hypertensinogenic activity in the rat". Hypertension. 27 (3 Pt 1): 421–5. doi:10.1161/01.hyp.27.3.421. PMID 8698448.
- 1 2 Gueux B, Fiet J, Galons H, Boneté R, Villette JM, Vexiau P, et al. (January 1987). "The measurement of 11 beta-hydroxy-4-pregnene-3,20-dione (21-deoxycorticosterone) by radioimmunoassay in human plasma". primary. Journal of Steroid Biochemistry. 26 (1): 145–50. doi:10.1016/0022-4731(87)90043-4. PMID 3546944.
- ↑ Fiet J, Gueux B, Raux-DeMay MC, Kuttenn F, Vexiau P, Brerault JL, et al. (March 1989). "Increased plasma 21-deoxycorticosterone (21-DB) levels in late-onset adrenal 21-hydroxylase deficiency suggest a mild defect of the mineralocorticoid pathway". primary. The Journal of Clinical Endocrinology and Metabolism. 68 (3): 542–7. doi:10.1210/jcem-68-3-542. PMID 2537337.
- 1 2 Fiet J, Le Bouc Y, Guéchot J, Hélin N, Maubert MA, Farabos D, Lamazière A (March 2017). "A Liquid Chromatography/Tandem Mass Spectometry [sic] Profile of 16 Serum Steroids, Including 21-Deoxycortisol and 21-Deoxycorticosterone, for Management of Congenital Adrenal Hyperplasia". primary. Journal of the Endocrine Society. 1 (3): 186–201. doi:10.1210/js.2016-1048. PMC 5686660. PMID 29264476.
- ↑ Nie M, Cui MX, Mao JF, Tong AL, Chen S, Wang X, et al. (December 2016). "[Possibility of progesterone as the diagnostic biomarker of 21-hydroxylase deficiency]". Zhonghua Yi Xue Za Zhi. 96 (48): 3866–3869. doi:10.3760/cma.j.issn.0376-2491.2016.48.003. PMID 28057154.
- ↑ Turcu AF, Auchus RJ (June 2015). "Adrenal steroidogenesis and congenital adrenal hyperplasia". Endocrinology and Metabolism Clinics of North America. 44 (2): 275–96. doi:10.1016/j.ecl.2015.02.002. PMC 4506691. PMID 26038201.
- ↑ Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ (2016). "Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency". Eur J Endocrinol. 174 (5): 601–9. doi:10.1530/EJE-15-1181. PMC 4874183. PMID 26865584.
- ↑ Auchus RJ, Buschur EO, Chang AY, Hammer GD, Ramm C, Madrigal D, Wang G, Gonzalez M, Xu XS, Smit JW, Jiao J, Yu MK (2014). "Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency". J Clin Endocrinol Metab. 99 (8): 2763–70. doi:10.1210/jc.2014-1258. PMC 4121028. PMID 24780050.
- ↑ Turcu AF, Mallappa A, Nella AA, Chen X, Zhao L, Nanba AT, Byrd JB, Auchus RJ, Merke DP (2021). "24-Hour Profiles of 11-Oxygenated C19 Steroids and Δ5-Steroid Sulfates during Oral and Continuous Subcutaneous Glucocorticoids in 21-Hydroxylase Deficiency". Front Endocrinol (Lausanne). 12: 751191. doi:10.3389/fendo.2021.751191. PMC 8636728. PMID 34867794.
- 1 2 Turcu AF, Mallappa A, Elman MS, Avila NA, Marko J, Rao H, Tsodikov A, Auchus RJ, Merke DP (2017). "11-Oxygenated Androgens Are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency". The Journal of Clinical Endocrinology and Metabolism. 102 (8): 2701–2710. doi:10.1210/jc.2016-3989. PMC 5546849. PMID 28472487.
- ↑ Schröder MAM, Turcu AF, O'Day P, van Herwaarden AE, Span PN, Auchus RJ, Sweep FCGJ, Claahsen-van der Grinten HL (2022). "Production of 11-Oxygenated Androgens by Testicular Adrenal Rest Tumors". J Clin Endocrinol Metab. 107 (1): e272–e280. doi:10.1210/clinem/dgab598. PMC 8684463. PMID 34390337.
- ↑ Masiutin M, Yadav M (2023). "Alternative androgen pathways". WikiJournal of Medicine. 10: X. doi:10.15347/WJM/2023.003. S2CID 257943362.
- ↑ Greaves RF, Kumar M, Mawad N, Francescon A, Le C, O'Connell M, Chi J, Pitt J (October 2023). "Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation". Int J Neonatal Screen. 9 (4): 58. doi:10.3390/ijns9040058. PMC 10594498. PMID 37873849.
- ↑ Cristoni S, Cuccato D, Sciannamblo M, Bernardi LR, Biunno I, Gerthoux P, et al. (2004). "Analysis of 21-deoxycortisol, a marker of congenital adrenal hyperplasia, in blood by atmospheric pressure chemical ionization and electrospray ionization using multiple reaction monitoring". primary. Rapid Communications in Mass Spectrometry. 18 (1): 77–82. Bibcode:2004RCMS...18...77C. doi:10.1002/rcm.1284. PMID 14689562.
- ↑ Sarathi V, Atluri S, Pradeep TV, Rallapalli SS, Rakesh CV, Sunanda T, Kumar KD (2019). "Utility of a Commercially Available Blood Steroid Profile in Endocrine Practice". primary. Indian Journal of Endocrinology and Metabolism. 23 (1): 97–101. doi:10.4103/ijem.IJEM_531_18. PMC 6446682. PMID 31016162.