 | |
 | |
| Names | |
|---|---|
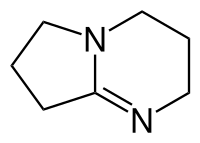
| Preferred IUPAC name
2,3,4,6,7,8-Hexahydropyrrolo[1,2-a]pyrimidine | |
| Other names
DBN | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.171 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H12N2 | |
| Molar mass | 124.18 g/mol |
| Density | 1.005 g/cm3 |
| Boiling point | 95 to 98 °C (203 to 208 °F; 368 to 371 K) at 7.5 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,5-Diazabicyclo[4.3.0]non-5-ene (DBN) is a chemical compound with the formula C7H12N2.[1] It is an amidine base used in organic synthesis. A related compound with related functions is 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The relatively complex nature of the formal names for DBU and DBN (hence the common use of acronyms) reflects the fact that these compounds are bicyclic and contain several functional groups.
Synthesis
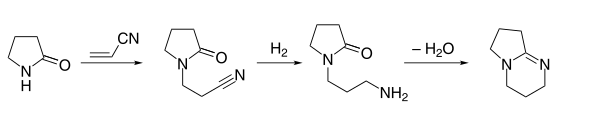
DBN could be synthesized in the following manner, similarly to DBU:[2]
The synthetic procedure starts with a Michael addition of 2-pyrrolidone to acrylonitrile, followed by hydrogenation, and finally dehydration.
Uses
As a base in organic synthesis
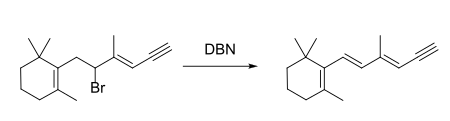
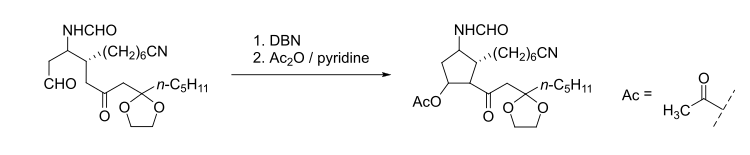
Similar to many other organic bases, DBN could be employed for dehydrohalogenation reactions, base-catalyzed rearrangement reactions, as well as Aldol condensation.[3] Several examples are shown below:
- Elimination:[2]
- Epimerization of penicillin derivatives, catalyzed by DBN:[4]
- Aldol condensation:[5]
DBN salt as an ionic liquid
The acetate salt of DBN is a room-temperature ionic liquid used for processing cellulose fibers by acting as a replacement for the unstable N-Methylmorpholine N-oxide used for making lyocell.[6][7]
References
- ↑ Savoca, Ann. C. "1,5-Diazabicyclo[4.3.0]non-5-ene" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rd010.pub2
- 1 2 Möller, F.; Oediger, H. "1,5-Diazabicyclo[5.4.0]undec-5-ene, a New Hydrogen Halide Acceptor" Angew. Chem. Int. Ed. Engl. , 1967, 5, 76. doi:10.1002/anie.196700761
- ↑ Oediger, H., Möller, F., & Eiter, K. "Bicyclic Amidines as Reagents in Organic Syntheses" Synthesis, 1972, 11, 591–598. doi:10.1055/s-1972-21943
- ↑ Jackson, J.R. and Stoodley, R.J. "Equilibration of penicillanic acid derivatives" J. Chem. Soc. D, 1971, 647-648
- ↑ Corey, E.J., Andersen, N.H., Carlson, R.M. "Total synthesis of prostaglandins. Synthesis of the pure dl-E1, -F1α, -F1β, -A1, and -B1 hormones" J. Am. Chem. Soc., 1968, 90, 12, 3245–3247 doi:10.1021/ja01014a053
- ↑ Zhang, Jinming; Wu, Jin; Yu, Jian; Zhang, Xiaoyu; He, Jiasong; Zhang, Jun. "Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends." Mat. Chem. Front. 2017, 1 (7), 1273-90. doi:10.1039/C6QM00348F
- ↑ Ioncell - Enter the new era of textile production!