 | |
 | |
| Names | |
|---|---|
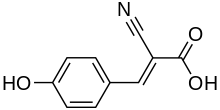
| Preferred IUPAC name
(2E)-2-Cyano-3-(4-hydroxyphenyl)prop-2-enoic acid | |
| Other names
α-Cyano-p-hydroxycinnamic acid 2-Cyano-4-hydroxycinnamic acid 2-Cyano-3-(4-hydroxyphenyl)-2-propenoic acid 2-Cyano-3-(4-hydroxyphenyl)-2-acrylic acid 4-Hydroxy-α-cyanocinnamic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.044.421 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H7NO3 | |
| Molar mass | 189.170 g·mol−1 |
| Appearance | Yellow powder |
| Melting point | 245 to 250 °C (473 to 482 °F; 518 to 523 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
α-Cyano-4-hydroxycinnamic acid, also written as alpha-cyano-4-hydroxycinnamic acid and abbreviated CHCA or HCCA, is a cinnamic acid derivative and is a member of the phenylpropanoid family. The carboxylate form is α-cyano-4-hydroxycinnamate.
Matrix-assisted laser desorption/ionization
α-Cyano-4-hydroxycinnamic acid is used as a matrix for peptides and nucleotides in MALDI mass spectrometry analyses.[1][2]
See also
References
- ↑ Beavis, R. C.; Chaudhary, T.; Chait, B. T. (1992). "-α-Cyano-4-hydroxycinnamic acid as a matrix for matrix-assisted laser desorption mass spectrometry". Org. Mass Spectrom. 27 (2): 156–8. doi:10.1002/oms.1210270217.
- ↑ Franz Hillenkamp; Jasna Peter-Katalinic (3 October 2013). MALDI MS: A Practical Guide to Instrumentation, Methods and Applications. Wiley. pp. 110–. ISBN 978-3-527-67373-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.