 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylmethanamine N-oxide | |
| Other names
Trimethylamine oxide, TMAO, TMANO | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.341 |
| KEGG | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H9NO | |
| Molar mass | 75.11 |
| Appearance | colorless solid |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) (dihydrate: 96 °C) |
| good | |
| 5.4 D | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
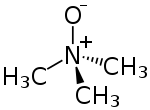
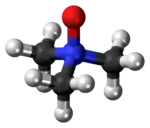
Trimethylamine N-oxide (TMAO) is an organic compound with the formula (CH3)3NO. It is in the class of amine oxides. Although the anhydrous compound is known, trimethylamine N-oxide is usually encountered as the dihydrate. Both the anhydrous and hydrated materials are white, water-soluble solids.
TMAO is found in the tissues of marine crustaceans and marine fish, where it prevents water pressure from distorting proteins and thus killing the animal. The concentration of TMAO increases with the depth at which the animal lives; TMAO is found in high concentrations in the deepest-living described fish species, Pseudoliparis swirei, which was found in the Mariana Trench, at a recorded depth of 8,076 m (26,496 ft).[1][2]
TMAO is a product of the oxidation of trimethylamine, a common metabolite of choline in animals.[3]
Marine animals
Trimethylamine N-oxide is an osmolyte found in molluscs, crustaceans, and all marine fishes and bony fishes. It is a protein stabilizer that serves to counteract the protein-destabilizing effects of pressure. In general, the bodies of animals living at great depths are adapted to high pressure environments by having pressure-resistant biomolecules and small organic molecules present in their cells, known as piezolytes, of which TMAO is the most abundant. These piezolytes give the proteins the flexibility they need to function properly under great pressure.[1][2][4][5][6]
TMAO decomposes to trimethylamine (TMA), which is the main odorant that is characteristic of degrading seafood.
Chemistry
TMAO can be synthesized from trimethylamine by treatment with hydrogen peroxide:[7]
- H2O2 + (CH3)3N → H2O + (CH3)3NO
The dihydrate is dehydrated by azeotropic distillation from dimethylformamide.[8]
Laboratory applications
Trimethylamine oxide is used in protein folding experiments to counteract the unfolding effects of urea.[9]
In the organometallic chemistry reaction of nucleophilic abstraction, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
- M(CO)n + Me3NO + L → M(CO)n−1L + Me3N + CO2
This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.[7]
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the corresponding aldehyde.[10]
Effects on protein stability
The effects of TMAO on the backbone and charged residues of peptides are found to stabilize compact conformations,[11] whereas effects of TMAO on nonpolar residues lead to peptide swelling. This suggests competing mechanisms of TMAO on proteins, which accounts for hydrophobic swelling, backbone collapse, and stabilization of charge-charge interactions. These mechanisms are observed in Trp cage.[12]
Disorders
Trimethylaminuria
Trimethylaminuria is a rare defect in the production of the enzyme flavin-containing monooxygenase 3 (FMO3).[13][14] Those suffering from trimethylaminuria are unable to convert choline-derived trimethylamine into trimethylamine oxide. Trimethylamine then accumulates and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
Cardiovascular disease
High TMAO concentrations have been suggested to be associated with an increased risk of cardiovascular disease and all-cause mortality.[15][16] However, it is the precursor, TMA and not TMAO, which has negative effects on cardiomyocytes, probably because of its cytotoxic effect on proteins, demonstrated experimentally in rats.[17]
Potential toxicity
Due to the widespread use of TMA in industry, various exposure limit guidelines with a detailed description of toxicity are available such as "Recommendation from the Scientific Committee on Occupational Exposure Limits" by the European Union Commission.[18]
References
- 1 2 Linley, T.D.; M.E. Gerringer; P.H. Yancey; J.C. Drazen; C.L. Weinstock; A.J. Jamieson (2016). "Fishes of the hadal zone including new species, in situ observations and depth records of Liparidae". Deep Sea Research Part I: Oceanographic Research Papers. 114: 99–110. Bibcode:2016DSRI..114...99L. doi:10.1016/j.dsr.2016.05.003.
- 1 2 Gerringer, M.E.; T.D. Linley; P.H. Yancey; A.J. Jamieson; E. Goetze; J.C. Drazen (2016). "Pseudoliparis swirei sp. nov.: A newly-discovered hadal snailfish (Scorpaeniformes: Liparidae) from the Mariana Trench". Zootaxa. 4358 (1): 161–177. doi:10.11646/zootaxa.4358.1.7. PMID 29245485.
- ↑ Baker, J.R.; Chaykin, S. (1 April 1962). "The biosynthesis of trimethylamine-N-oxide". J. Biol. Chem. 237 (4): 1309–13. doi:10.1016/S0021-9258(18)60325-4. PMID 13864146.
- ↑ Yancey, P. (2005). "Organic osmolytes as compatible, metabolic, and counteracting cytoprotectants in high osmolarity and other stresses". J. Exp. Biol. 208 (15): 2819–2830. doi:10.1242/jeb.01730. PMID 16043587.
- ↑ Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. (8 November 2016). "Trimethylamine N-Oxide: The good, the bad and the unknown". Toxins. 8 (11): 326. doi:10.3390/toxins8110326. PMC 5127123. PMID 27834801.
- ↑ "What does it take to live at the bottom of the ocean?". BBC Earth. 2016. Archived from the original on 13 May 2016. Retrieved 19 May 2016.
- 1 2 A. J. Pearson "Trimethylamine N-Oxide" in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2001: New York. doi:10.1002/047084289X.rt268
- ↑ Soderquist, J. A.; Anderson, C. L. (1986). "Crystalline anhydrous trimethylamine N-oxide". Tetrahedron Lett. 27 (34): 3961–3962. doi:10.1016/S0040-4039(00)84884-4.
- ↑ Zou, Q.; et al. (2002). "The Molecular Mechanism of Stabilization of Proteins by TMAO and Its Ability to Counteract the Effects of Urea". J. Am. Chem. Soc. 124 (7): 1192–1202. doi:10.1021/ja004206b. PMID 11841287.
- ↑ Volker Franzen (1973). "Octanal". Organic Syntheses.; Collective Volume, vol. 5, p. 872
- ↑ Shea, Joan-Emma; Feinstein, Stuart C.; Lapointe, Nichole E.; Larini, Luca; Levine, Zachary A. (2015-03-03). "Regulation and aggregation of intrinsically disordered peptides". Proceedings of the National Academy of Sciences of the United States of America. 112 (9): 2758–2763. Bibcode:2015PNAS..112.2758L. doi:10.1073/pnas.1418155112. PMC 4352815. PMID 25691742.
- ↑ Su, Zhaoqian; Mahmoudinobar, Farbod; Dias, Cristiano L. (2017). "Effects of Trimethylamine-N-oxide on the Conformation of Peptides and its Implications for Proteins". Physical Review Letters. 119 (10): 108102. Bibcode:2017PhRvL.119j8102S. doi:10.1103/physrevlett.119.108102. PMID 28949191.
- ↑ Treacy, E.P.; Akerman, BR; et al. (1998). "Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication". Human Molecular Genetics. 7 (5): 839–45. doi:10.1093/hmg/7.5.839. PMID 9536088.
- ↑ Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E (1999). "Mild trimethylaminuria caused by common variants in FMO3 gene". Lancet. 354 (9181): 834–5. doi:10.1016/S0140-6736(99)80019-1. PMID 10485731. S2CID 9555588.
- ↑ Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. (2017). "Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis". European Heart Journal. 38 (39): 2948–2956. doi:10.1093/eurheartj/ehx342. PMID 29020409.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Guasti L, Galliazzo S, Molaro M, Visconti E, Pennella B, Gaudio GV, Lupi A, Grandi AM, Squizzato A. (2021). "TMAO as a biomarker of cardiovascular events: a systematic review and meta-analysis". Intern Emerg Med. 16 (1): 201–207. doi:10.1007/s11739-020-02470-5. PMID 32779113. S2CID 221099557.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Jaworska, Kinga; Hering, Dagmara; Mosieniak, Grażyna; Bielak-Zmijewska, Anna; Pilz, Marta; Konwerski, Michał; Gasecka, Aleksandra; Kapłon-Cieślicka, Agnieszka; Filipiak, Krzysztof; Sikora, Ewa; Hołyst, Robert; Ufnal, Marcin (2019-08-26). "TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology". Toxins. 11 (9): 490. doi:10.3390/toxins11090490. ISSN 2072-6651. PMC 6784008. PMID 31454905.
- ↑ Directorate-General for Employment, Social Affairs and Inclusion (European Commission); Scientific Committee on Occupational Exposure Limits; Nielsen, G. D.; Pospischil, E.; Johanson, G.; Klein, C. L.; Papameletiou, D. (2017). SCOEL/REC/179 trimethylamine: recommendation from the Scientific Committee on Occupational Exposure Limits. Publications Office of the European Union. doi:10.2767/440659. ISBN 9789279666278. Retrieved 2023-12-17.