| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propanamide | |||
| Other names
n-propylamide Propionamide Propylamide Propionic amide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.066 | ||
| EC Number |
| ||
| MeSH | C034666 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H7NO | |||
| Molar mass | 73.095 g·mol−1 | ||
| Appearance | liquid , yellow | ||
| Density | 1.042 g/mL | ||
| Melting point | 80 °C (176 °F; 353 K) | ||
| Boiling point | 213 °C (415 °F; 486 K) | ||
| very soluble in water | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
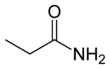
Propanamide has the chemical formula CH3CH2C=O(NH2).[1] It is the amide of propanoic acid.
This organic compound is a mono-substituted amide.[2] Organic compounds of the amide group can react in many different organic processes to form other useful compounds for synthesis.
Preparation
Propanamide can be prepared by the condensation reaction between urea and propanoic acid:
or by the dehydration of ammonium propionate:
Reactions
Propanamide being an amide can participate in a Hoffman rearrangement to produce ethylamine gas.
References
- ↑ Ramazani, Ali; Rouhani, Morteza; Joo, Sang Woo (2016-01-01). "Catalyst-free sonosynthesis of highly substituted propanamide derivatives in water". Ultrasonics Sonochemistry. 28: 393–399. doi:10.1016/j.ultsonch.2015.08.019. ISSN 1350-4177. PMID 26384923.
- ↑ Ye, Xuewei; Chai, Weiyun; Lian, Xiao-Yuan; Zhang, Zhizhen (2017-06-18). "Novel propanamide analogue and antiproliferative diketopiperazines from mangrove Streptomyces sp. Q24". Natural Product Research. 31 (12): 1390–1396. doi:10.1080/14786419.2016.1253079. ISSN 1478-6419. PMID 27806640. S2CID 24563632.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.