 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60 to 65% |
| Elimination half-life | 0.4 hours |
| Excretion | In feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.168.193 |
| Chemical and physical data | |
| Formula | C19H18N4O2 |
| Molar mass | 334.379 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Pimobendan (INN, or pimobendane; trade names Vetmedin, Acardi) is a veterinary medication. It is a calcium sensitizer and a selective inhibitor of phosphodiesterase 3 (PDE3) with positive inotropic and vasodilator effects.
Pimobendan is used in the management of heart failure in dogs, most commonly caused by myxomatous mitral valve disease (also previously known as endocardiosis), or dilated cardiomyopathy.[1] Research has shown that as a monotherapy, pimobendan increases survival time and improves quality of life in canine patients with congestive heart failure secondary to mitral valve disease when compared with benazepril, an ACE inhibitor.[2] However, in clinical practice, it is often used in conjunction with an ACE inhibitor like enalapril or benazepril. Under the trade name Acardi, it is available for human use in Japan.[3]
Mechanism of action
Pimobendan is a positive inotrope (increases myocardial contractility). It sensitizes and increases the binding efficiency of cardiac troponin in the myofibril to the calcium ions that are already present in systole. In normal hearts it increases the consumption of oxygen and energy to the same degree as dobutamine but in diseased hearts it may not.[4][5] Pimobendan also causes peripheral vasodilation by inhibiting the function of PDE3. This results in decreased resistance to blood flow through systemic arterioles, which decreases afterload (decreases the failing heart's workload) and reduces the amount of mitral regurgitation.[6][7]
Pharmacokinetics
Pimobendan is absorbed rapidly when given via the oral route and has a bioavailability of 60-65%.[8] Bioavailability is markedly decreased when ingested with food. It is metabolized into an active metabolite (desmethylpimobendan) by the liver. The parent compound, pimobendan, is a potent calcium sensitizer while desmethylpimobendan is a more potent phosphodiesterase III inhibitor.[9] The half-life of pimobendan in the blood is 0.4 hours, and the half-life of its metabolite is two hours. Elimination is by excretion in the bile and then feces. Pimobendan is 90–95% bound to plasma proteins in circulation. This may have implications in patients with low blood protein levels (hypoproteinemia/hypoalbuminemia) and in patients that are on concurrent therapies that are also highly protein bound.
Combinations
Pimobendan is often used in combination with three other drugs to palliate dogs with heart failure (pulmonary edema, pleural effusion, ascites). These are:
- Furosemide, a diuretic, to reduce edema and effusion.
- Spironolactone, an aldosterone antagonist. This has two actions, firstly, as a potassium-sparing diuretic, although its diuretic properties are small compared with those of furosemide. Secondly, it reduces aldosterone-mediated myocardial fibrosis, possibly slowing the progression of heart disease.
- An ACE inhibitor, often enalapril (trade name Enacard) or benazepril (Fortekor). These drugs inhibit the action of angiotensin-converting enzyme, producing a balanced vasodilation, along with other potentially favorable effects.
Other drugs may also be used as required to manage certain arrhythmias that are often associated with heart disease.
Synthesis
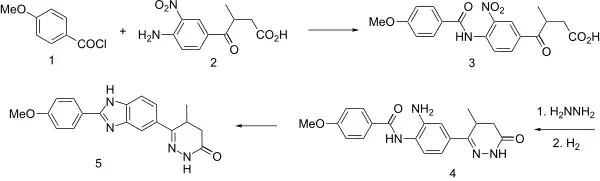
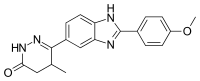
The reaction between p-anisoyl chloride [100-07-2] (1) and CID:20516917 (2) gives 4-[4-[(4-Methoxybenzoyl)amino]-3-nitrophenyl]-3-methyl-4-oxobutanoic acid, CID:20516902 (3). The reaction of this with hydrazine gives 5-methyl-6-[3-nitro-4-(4-methoxy-benzoylamino)-phenyl]-3-oxo-4,5-dihydro-2H-pyridazine [74149-73-8]. Catalytic hydrogenation reduces the nitro group giving [74149-74-9] (4). cyclization of the resulting ortho amino amide by means of a strong acid leads to the formation of the corresponding benzimidazole. There is thus obtained pimobendan (5).
See also
References
- ↑ Gordon SG, Miller MW, Saunders AB (2006). "Pimobendan in heart failure therapy—a silver bullet?". J Am Anim Hosp Assoc. 42 (2): 90–3. doi:10.5326/0420090. PMID 16527909.
- ↑ Häggström J, Boswood A, O'Grady M, et al. (July 2008). "Effect of Pimobendan or Benazepril Hydrochloride on Survival Times in Dogs with Congestive Heart Failure Caused by Naturally Occurring Myxomatous Mitral Valve Disease: The QUEST Study". J. Vet. Intern. Med. 22 (5): 1124–35. CiteSeerX 10.1.1.661.3009. doi:10.1111/j.1939-1676.2008.0150.x. PMID 18638016.
- ↑ "Kusuri-no-Shiori Drug Information Sheet". RAD-AR Council, Japan. April 2005. Retrieved 2008-08-06.
- ↑ Hata K1, Goto Y, Futaki S, Ohgoshi Y, Yaku H, Kawaguchi O, Takasago T, Saeki A, Taylor TW, Nishioka T, et al. "Mechanoenergetic effects of pimobendan in canine left ventricles. Comparison with dobutamine." Circulation. 1992 Oct;86(4):1291-301.
- ↑ Goto Y1, Hata K. Mechanoenergetic effect of pimobendan in failing dog hearts. Heart Vessels. 1997;Suppl 12:103-5.
- ↑ Verdouw PD, Hartog JM, Duncker DJ, Roth W, Saxena PR. "Cardiovascular profile of pimobendan, a benzimidazole-pyridazinone derivative with vasodilating and inotropic properties." Eur J Pharmacol. 1986 Jul 15;126(1-2):21-30.
- ↑ Kanno N, Kuse H, Kawasaki M, Hara A, Kano R, Sasaki Y. "Effects of pimobendan for mitral valve regurgitation in dogs." J Vet Med Sci. 2007 Apr;69(4):373-7.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2015-02-06. Retrieved 2014-12-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Hanzlicek AS1, Gehring R, Kukanich B, Kukanich KS, Borgarelli M, Smee N, Olson EE, Margiocco M. "Pharmacokinetics of oral pimobendan in healthy cats." J Vet Cardiol. 2012 Dec;14(4):489-96.
- ↑ Volkhard Dipl Chem Dr Austel, 4 More », DE 2837161 (1981 to Thomae Gmbh Dr K).
- ↑ Volkhard Austel, Joachim Heider, Wolfgang Eberlein, Willi Diederen, Walter Haarmann, U.S. Patent 4,361,563 (1982 to Dr. Karl Thomae Gesellschaft Mit Beschrankter Haftung).
- ↑ Marinus Maria Martinus BOEREN, 4 More », US 20110152283 (2011 to Eurovet Animal Health B.V.).
- ↑ Christian Schickaneder, 4 More », WO 2011124638 (2011 to Novartis Ag).
- ↑ Cao Lei, et al. CN 105968054 (2016 to Wisdom Pharmaceutical Co Ltd).
Further reading
- Lee JA, Allen DG (March 1990). "Calcium sensitisers". BMJ. 300 (6724): 551–2. doi:10.1136/bmj.300.6724.551. PMC 1662365. PMID 2108746.