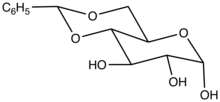
Benzylidene compounds are, formally speaking, derivatives of benzylidene, although few are prepared from the carbene. Benzylidene acetal is a protecting group in synthetic organic chemistry of the form PhCH(OR)2. For example, 4,6-O-benzylidene-glucopyranose is a glucose derivative. Benzylidene is an archaic term for compounds of the type PhCHX2 and PhCH= substituents (Ph = C6H5). For example, dibenzylideneacetone is (PhCH=CH)2CO. Benzal chloride, PhCHCl2, is alternatively named benzylidene chloride.
Benzylidene is the molecule C6H5CH. It is a triplet carbene (CAS RN 3101-08-4). It is generated by irradiation of phenyldiazomethane.[1]
See also
References
- ↑ Platz, Matthew S. (1995). "Comparison of Phenylcarbene and Phenylnitrene". Accounts of Chemical Research. 28 (12): 487–92. doi:10.1021/ar00060a004.
External links
Look up benzylidene in Wiktionary, the free dictionary.
- Benzylidene+Compounds at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.