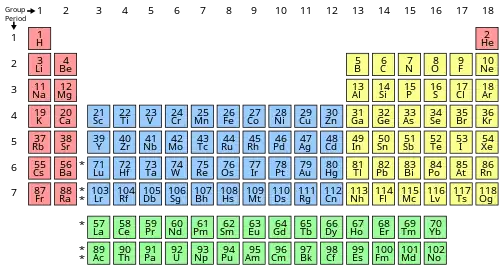
In chemistry, a group (also known as a family)[1] is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the 14 f-block columns, between groups 2 and 3, are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because most chemical properties are dominated by the orbital location of the outermost electron.
There are three systems of group numbering for the groups; the same number may be assigned to different groups depending on the system being used. The modern numbering system of "group 1" to "group 18" has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since 1988. It replaces two older incompatible naming schemes, used by the Chemical Abstract Service (CAS, more popular in the United States), and by IUPAC before 1988 (more popular in Europe). The system of eighteen groups is generally accepted by the chemistry community, but some dissent exists about membership of elements number 1 and 2 (hydrogen and helium). Similar variation on the inner transition metals continues to exist in textbooks, although the correct positioning has been known since 1948 and was twice endorsed by IUPAC in 1988 (together with the 1–18 numbering) and 2021.
Groups may also be identified using their topmost element, or have a specific name. For example, group 16 is also described as the "oxygen group" and as the "chalcogens". An exception is the "iron group", which usually refers to group 8, but in chemistry may also mean iron, cobalt, and nickel, or some other set of elements with similar chemical properties. In astrophysics and nuclear physics, it usually refers to iron, cobalt, nickel, chromium, and manganese.
Group names
Modern group names are numbers 1–18, with the 14 f-block columns remaining unnumbered (together making the 32 columns in the periodic table). Also, trivial names (like halogens) are common. In history, several sets of group names have been used, based on Roman numberings I–VIII, and "A" and "B" suffixes.[2][3]
| IUPAC group | 1a | 2 | —b | 3c | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mendeleev (I–VIII) | I | II | III | IV | V | VI | VII | VIII | I | II | III | IV | V | VI | VII | d | |||
| CAS (US, A-B-A) | IA | IIA | IIIB | IVB | VB | VIB | VIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA | VIIIA | |||
| Old IUPAC (Europe, A-B) | IA | IIA | IIIA | IVA | VA | VIA | VIIA | VIII | IB | IIB | IIIB | IVB | VB | VIB | VIIB | 0 | |||
| Trivial namer | H and alkali metals | alkaline earth metals | triels | tetrels | pnictogens | chalcogens | halogens | noble gases | |||||||||||
| Name by elementr | lithium group | beryllium group | scandium group | titanium group | vanadium group | chromium group | manganese group | iron group | cobalt group | nickel group | copper group | zinc group | boron group | carbon group | nitrogen group | oxygen group | fluorine group | helium or neon group | |
| Period 1 | H | He | |||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| Period 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Period 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Period 6 | Cs | Ba | La–Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Period 7 | Fr | Ra | Ac–No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
b The 14 f-block groups (columns) do not have a group number.
c The correct composition of group 3 is scandium (Sc), yttrium (Y), lutetium (Lu), and lawrencium (Lr), as shown here: this is endorsed by 1988[4] and 2021[5] IUPAC reports on the question. General inorganic chemistry texts often put scandium (Sc), yttrium (Y), lanthanum (La), and actinium (Ac) in group 3, so that Ce–Lu and Th–Lr become the f-block between groups 3 and 4; this was based on incorrectly measured electron configurations from history,[6] and Lev Landau and Evgeny Lifshitz already considered it incorrect in 1948.[7] Arguments can still occasionally be encountered in the contemporary literature purporting to defend it, but most authors consider them logically inconsistent.[8][9][10] Some sources follow a compromise that puts La–Lu and Ac–Lr as the f-block rows (despite that giving 15 f-block elements in each row, which contradicts quantum mechanics), leaving the heavier members of group 3 ambiguous.[5] See also Group 3 element#Composition.
d Group 18, the noble gases, were not discovered at the time of Mendeleev's original table. Later (1902), Mendeleev accepted the evidence for their existence, and they could be placed in a new "group 0", consistently and without breaking the periodic table principle.
r Group name as recommended by IUPAC.
List of group names
| IUPAC name |
Old IUPAC (Europe) |
Old CAS name (U.S.) |
Name by element ('group' or 'family') |
IUPAC recommended trivial name |
Other names |
|---|---|---|---|---|---|
| Group 1 | IA | IA | lithium group | hydrogen and alkali metals | "lithium group" excludes hydrogen |
| Group 2 | IIA | IIA | beryllium group | alkaline earth metals | |
| Group 3 | IIIA | IIIB | scandium group | ||
| Group 4 | IVA | IVB | titanium group | ||
| Group 5 | VA | VB | vanadium group | ||
| Group 6 | VIA | VIB | chromium group | ||
| Group 7 | VIIA | VIIB | manganese group | ||
| Group 8 | VIII | VIIIB | iron group | ||
| Group 9 | VIII | VIIIB | cobalt group | ||
| Group 10 | VIII | VIIIB | nickel group | ||
| Group 11 | IB | IB | copper group | Sometimes called coinage metals, but the set is arbitraryf | |
| Group 12 | IIB | IIB | zinc group | volatile metals[11] | |
| Group 13 | IIIB | IIIA | boron group | trielsb | icosagens[12] earth metals |
| Group 14 | IVB | IVA | carbon group | tetrelsc | crystallogens[13] adamantogens[14] merylides[15] |
| Group 15 | VB | VA | nitrogen group | pnictogens pentelsn |
|
| Group 16 | VIB | VIA | oxygen group | chalcogens | |
| Group 17 | VIIB | VIIA | fluorine group | halogens | |
| Group 18 | 0 | VIIIA | helium group or neon group |
noble gases | aerogens[16] |
- ^f Coinage metals: authors differ on whether roentgenium (Rg) is considered a coinage metal. It is in group 11, like the other coinage metals, and is expected to be chemically similar to gold.[17] On the other hand, being extremely radioactive and short-lived, it cannot actually be used for coinage as the name suggests, and on that basis it is sometimes excluded.[18]
CAS and old IUPAC numbering (A/B)
Two earlier group number systems exist: CAS (Chemical Abstracts Service) and old IUPAC. Both use numerals (Arabic or Roman) and letters A and B. Both systems agree on the numbers. The numbers indicate approximately the highest oxidation number of the elements in that group, and so indicate similar chemistry with other elements with the same numeral. The number proceeds in a linearly increasing fashion for the most part, once on the left of the table, and once on the right (see List of oxidation states of the elements), with some irregularities in the transition metals. However, the two systems use the letters differently. For example, potassium (K) has one valence electron. Therefore, it is located in group 1. Calcium (Ca) is in group 2, for it contains two valence electrons.
In the old IUPAC system the letters A and B were designated to the left (A) and right (B) part of the table, while in the CAS system the letters A and B are designated to main group elements (A) and transition elements (B). The old IUPAC system was frequently used in Europe, while the CAS is most common in America. The new IUPAC scheme was developed to replace both systems as they confusingly used the same names to mean different things. The new system simply numbers the groups increasingly from left to right on the standard periodic table. The IUPAC proposal was first circulated in 1985 for public comments,[2] and was later included as part of the 1990 edition of the Nomenclature of Inorganic Chemistry.[19]
Non-columnwise groups
While groups are defined to be columns in the periodic table, as described above, there are also sets of elements named "group" that are not a column:
Similar sets: noble metals, coinage metals, precious metals, refractory metals.
References
- ↑ "The Periodic Table Terms". www.shmoop.com. Retrieved 2018-09-15.
- 1 2 Fluck, E. (1988). "New Notations in the Periodic Table" (PDF). Pure Appl. Chem. IUPAC. 60 (3): 431–436. doi:10.1351/pac198860030431. S2CID 96704008. Retrieved 24 March 2012.
- ↑ IUPAC (2005). "Nomenclature of inorganic chemistry" (PDF).
- ↑ Fluck, E. (1988). "New Notations in the Periodic Table" (PDF). Pure Appl. Chem. 60 (3): 431–436. doi:10.1351/pac198860030431. S2CID 96704008. Archived (PDF) from the original on 25 March 2012. Retrieved 24 March 2012.
- 1 2 Scerri, Eric (18 January 2021). "Provisional Report on Discussions on Group 3 of the Periodic Table" (PDF). Chemistry International. 43 (1): 31–34. doi:10.1515/ci-2021-0115. S2CID 231694898. Archived (PDF) from the original on 13 April 2021. Retrieved 9 April 2021.
- ↑ William B. Jensen (1982). "The Positions of Lanthanum (Actinium) and Lutetium (Lawrencium) in the Periodic Table". J. Chem. Educ. 59 (8): 634–636. Bibcode:1982JChEd..59..634J. doi:10.1021/ed059p634.
- ↑ L. D. Landau, E. M. Lifshitz (1958). Quantum Mechanics: Non-Relativistic Theory. Vol. 3 (1st ed.). Pergamon Press. pp. 256–7.
- ↑ Jensen, William B. (2015). "The positions of lanthanum (actinium) and lutetium (lawrencium) in the periodic table: an update". Foundations of Chemistry. 17: 23–31. doi:10.1007/s10698-015-9216-1. S2CID 98624395. Archived from the original on 30 January 2021. Retrieved 28 January 2021.
- ↑ Scerri, Eric (2009). "Which Elements Belong in Group 3?". Journal of Chemical Education. 86 (10): 1188. doi:10.1021/ed086p1188. Retrieved 1 January 2023.
- ↑ Chemey, Alexander T.; Albrecht-Schmitt, Thomas E. (2019). "Evolution of the periodic table through the synthesis of new elements". Radiochimica Acta. 107 (9–11): 1–31. doi:10.1515/ract-2018-3082.
- ↑ Simmons, L. M. (1947). "A modification of the periodic table". Journal of Chemical Education. 24 (12): 588–591. doi:10.1021/ed024p588.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 227. ISBN 978-0-08-037941-8.
- 1 2 3 Liu, Ning; Lu, Na; Su, Yan; Wang, Pu; Quan, Xie (2019). "Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation". Separation and Purification Technology. 211: 782–789. doi:10.1016/j.seppur.2018.10.027. S2CID 104746665. Retrieved 17 August 2019.
- ↑ Jensen, William B. (2000). "The Periodic Law and Table" (PDF). Archived from the original (PDF) on 2020-11-10. Retrieved 10 December 2022.
- ↑ Fernelius, W. C.; Loening, Kurt; Adams, Roy M. (1971). "Names of groups and elements". Journal of Chemical Education. 48 (11): 730–731. doi:10.1021/ed048p730.
- 1 2 3 4 Rich, Ronald (2007). Inorganic Reactions in Water. Springer. pp. 307, 327, 363, 475. doi:10.1007/978-3-540-73962-3. ISBN 9783540739616.
- ↑ Conradie, Jeanet; Ghosh, Abhik (2019). "Theoretical Search for the Highest Valence States of the Coinage Metals: Roentgenium Heptafluoride May Exist". Inorganic Chemistry. 58 (13): 8735–8738. doi:10.1021/acs.inorgchem.9b01139. PMID 31203606. S2CID 189944098.
- ↑ Grochala, Wojciech; Mazej, Zoran (2015). "Chemistry of silver(II): a cornucopia of peculiarities". Philosophical Transactions of the Royal Society A. 373 (2037). doi:10.1098/rsta.2014.0179. PMID 25666068. S2CID 45589426.
- ↑ Leigh, G. J. Nomenclature of Inorganic Chemistry: Recommendations 1990. Blackwell Science, 1990. ISBN 0-632-02494-1.
Further reading
- Scerri, E. R. (2007). The periodic table, its story and its significance. Oxford University Press. ISBN 978-0-19-530573-9.