 | |
| Names | |
|---|---|
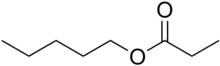
| Preferred IUPAC name
Pentyl propanoate | |
| Other names
Pentyl propionate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.866 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H16O2 | |
| Molar mass | 144.22 g/mol |
| Appearance | Sweet fruity odor of apricot pineapple [1] |
| Density | 0.870 g/cm3 |
| Melting point | −75 °C (−103 °F; 198 K) |
| Boiling point | 168 °C (334 °F; 441 K) |
| Related compounds | |
Related Esters |
Propyl propanoate Butyl propanoate Hexyl propanoate Pentyl acetate Pentyl butanoate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Pentyl propanoate (also known as amyl propionate) is an organic ester formed by the condensation of pentan-1-ol and propanoic acid.[2] It is a colorless liquid with an apple-like odor, that floats on water.[3]
References
- ↑ Reference Book on Fragrance Ingredients.pdf A Reference Book on Fragrance Ingredients
- ↑ "Amyl Propionate". Chemland21. Retrieved 15 December 2012.
- ↑ "N-Pentyl Propionate". CAMEO Chemicals. NOAA. Retrieved 15 December 2012.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.