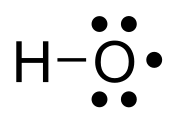 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hydroxyl radical | |||
| Systematic IUPAC name | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| 105 | |||
| KEGG | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| HO | |||
| Molar mass | 17.007 g·mol−1 | ||
| Thermochemistry | |||
Std molar entropy (S⦵298) |
183.71 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
38.99 kJ mol−1 | ||
| Related compounds | |||
Related compounds |
O2H+ OH− O22− | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
The hydroxyl radical is the diatomic molecule •
OH. The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations.[2] Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O.[3]
Structure and bonding
The O-H distance is 0.97 Å. The O-H vibrational frequency is 3570 cm-1. These data are very similar to those for water.[4] The radical resides on the oxygen atom. With nine electrons, hydroxyl's electronic structure is described by (1s)2(2sσ)2(2pσ)2(2pπ)3.[5]
The electronic angular momentum along the molecular axis is +1 or −1, and the electronic spin angular momentum S = 1⁄2. Because of the orbit-spin coupling, the spin angular momentum can be oriented in parallel or anti parallel directions to the orbital angular momentum, producing the splitting into Π1⁄2 and Π3⁄2 states. The 2Π3⁄2 ground state of •OH is split by lambda doubling interaction (an interaction between the nuclei rotation and the unpaired electron motion around its orbit). Hyperfine interaction with the unpaired spin of the proton further splits the levels.
Production
Hydroxyl radicals can be generated in several ways.[6]
Photolysis of H2O2
Laser photolysis of hydrogen peroxide proceeds with a quantum yield of 0.4-0.5:
- H2O2 → 2 HO·
The recombination of hydroxyl radicals proceeds with a second order rate constant of 4.7 × 109 M−1s−1 (25 °C).
Hydroxyl radicals undergo a series of reactions with hydrogen peroxide, initially giving hydroperoxo radical:
- H2O2 + HO· → H2O + HO2·
and subsequently regenerating the hydroxyl radical together with oxygen:
- H2O2 + HO2· → H2O + O2 + HO·
Fenton reaction
The Fenton reaction produces hydroxyl radicals by this stoichiometry:
- H2O2 + Fe+2 → HO· + FeOH+2
In this equation Fe2+ is the aquo complex [Fe(H2O)6]2+ and FeOH2+ is a ferric derivative [Fe(OH)(H2O)5]2+. The reaction is often conduced at pH 3−4, and proceeds with concomitant rrecipitation of solid ferric hydroxide. Many variations of this process have been developed such as the use of ferrous complex of edta. The complexant edta allows one to generate hydroxyl radicals at pH 7, which is compatible with DNA and other biomolecules. This approach is used in DNA footprinting.[6]
Radiolysis
Pulse radiolysis of water produces hydroxyl radicals as well as solvated electrons.
- H2O → e− + H2O+
- H2O+ → HO· + H+
Quenching occurs by formation of hydrogen peroxide and hydroxide formation: HO· + e− → OH−
Chemical and biochemical reactions
The hydroxyl radical reacts at nearly diffusion rates with all organic compounds. Thus, it is the most dangerous member of the reactive oxygen species. In one manifestation of this reactivity, the hydroxyl radical contributes significantly in vivo to oxidative damage to DNA. The hydroxyl radical can cause numerous types of damage to the nucleotide bases of DNA (for example, formation of 8-Oxo-2'-deoxyguanosine), as well as deoxyribose damage, strand breaks and interstrand cross-links. Oxidative damage to DNA has an important role in the origin and progression of several human diseases, most prominently cancer, but also neurodegenerative diseases and atherosclerosis.[7]
Water purification
Hydroxyl radicals play a key role in the oxidative destruction of organic pollutants using a series of methodologies collectively known as advanced oxidation processes (AOPs). The destruction of pollutants in AOPs is based on the non-selective reaction of hydroxyl radicals on organic compounds. It is highly effective against a series of pollutants including pesticides, pharmaceutical compounds, dyes, etc.[8][9]
Atmosphere
Hydroxyl radical is pervasive in the atmosphere and its behavior governs much of atmospheric chemistry.[10] Although its lifetime is about 1 second, the hydroxyl •OH radical is reactive toward many components of the atmosphere including methane, sulfur dioxide, carbon monoxide, nitrogen oxides, and any organic compound.[11] Atmospheric hydroxyl is estimated to remove 3.7 gigatons of gases annually. In addition to the previously listed gases, other gases destroyed include HFCs and HCFC's.[12]
Understanding the role of •OH in the oxidation process of methane (CH4) present in the atmosphere to first carbon monoxide (CO) and then carbon dioxide (CO2) is important for assessing the residence time of this greenhouse gas, the overall carbon budget of the troposphere, and its influence on the process of global warming. The lifetime of •OH radicals in the Earth atmosphere is very short, therefore •OH concentrations in the air are very low and very sensitive techniques are required for its direct detection.[13] Global average hydroxyl radical concentrations have been measured indirectly by analyzing methyl chloroform (CH3CCl3) present in the air. The results obtained by Montzka et al. (2011)[14] shows that the interannual variability in •OH estimated from CH3CCl3 measurements is small, indicating that global •OH is generally well buffered against perturbations. This small variability is consistent with measurements of methane and other trace gases primarily oxidized by •OH, as well as global photochemical model calculations.
In 2014, researchers reported their discovery of a "hole" or absence of hydroxyl throughout the entire depth of the troposphere across a large region of the tropical West Pacific. They suggested that this hole is permitting large quantities of ozone-degrading chemicals to reach the stratosphere, and that this may be significantly reinforcing ozone depletion in the polar regions with potential consequences for the climate of the Earth.[15]
Astronomy
It is convenient to distinguish two types of interstellar clouds: diffuse clouds, with T = 30–100 K and n = 10–1000 cm−3, and dense clouds, with T = 10–30 K and density n = 104–103 cm−3.[16]
The first experimental evidence for the presence of 18 cm absorption lines of the hydroxyl (•OH) radical in the radio absorption spectrum of Cassiopeia A was obtained by Weinreb et al.[17] based on observations made during the period October 15–29, 1963.[18] Reports on interstellar hydroxyl radical continued in earnest in the 1960's and 1970's.[19][20][21][22][23][24][25]
Production pathways in the interstellar medium
The •OH radical is linked with the production of H2O in molecular clouds. Studies of •OH distribution in Taurus Molecular Cloud-1 (TMC-1)[26] suggest that in dense gas, •OH is mainly formed by dissociative recombination of H3O+. Dissociative recombination is the reaction in which a molecular ion recombines with an electron and dissociates into neutral fragments. Important formation mechanisms for •OH are:
-
H3O+ + e− → •OH + H2
(Dissociative recombination: 1a)
-
H3O+ + e− → •OH + •H + •H
(Dissociative recombination: 1b)
-
HCO+
2 + e− → •OH + CO(Dissociative recombination: 2a)
-
•O + HCO → •OH + CO
(Neutral–neutral: 3a)
-
H− + H3O+ → •OH + H2 + •H
(Ion–molecular ion neutralization: 4a)
Destruction pathways in the interstellar medium
Small neutral molecules in the interstellar clouds may be formed by reactions of •H and •OH.[27] The formation of O2 occurs in the gas phase via the neutral exchange reaction between O and •OH, which is also the main sink for •OH in dense regions.[26]
Atomic oxygen takes part both in the production and destruction of •OH, so the abundance of •OH depends mainly on the H3+ abundance. Then, important chemical pathways leading from •OH radicals are:
-
•OH + O → O2 + •H
(Neutral–neutral: 1A)
-
•OH + C+ → CO+ + •H
(Ion–neutral: 2A)
-
•OH + •N → NO + •H
(Neutral–neutral: 3A)
-
•OH + C → CO + •H
(Neutral–neutral: 4A)
-
•OH + •H → H2O + photon
(Neutral–neutral: 5A)
Rate constants and relative rates for important formation and destruction mechanisms
Rate constants can be derived from the dataset published in a website.[28] Rate constants have the form:
- k(T) = α(T/300)β × exp(−γ/T) cm3 s−1
The following table has the rate constants calculated for a typical temperature in a dense cloud T = 10 K.
Reaction k at T = 10 K (cm3·s−1) 1a 3.29×10−6 1b 1.41×10−7 2a 4.71×10−7 3a 5.0×10−11 4a 1.26×10−6 5a 2.82×10−6 1A 7.7×10−10 2A 3.5×10−11 3A 1.38×10−10 4A 1.0×10−10 5A 3.33×10−14
Formation rates rix can be obtained using the rate constants k(T) and the abundances of the reactants species C and D:
- rix = k(T)ix[C][D]
where [Y] represents the abundance of the species Y. In this approach, abundances were taken from The UMIST database for astrochemistry 2006, and the values are relatives to the H2 density. The following table shows the ratio rix/r1a in order to get a view of the most important reactions.
The results suggest that 1a reaction is the most prominent reaction in dense clouds. It is in concordance with Harju et al. 2000.
The next table shows the results by doing the same procedure for the destruction reaction:
The results show that reaction 1A is the main sink for •OH in dense clouds.
Interstellar observations
Discoveries of the microwave spectra of a considerable number of molecules prove the existence of rather complex molecules in the interstellar clouds, and provides the possibility to study dense clouds, which are obscured by the dust they contain.[29] The •OH molecule has been observed in the interstellar medium since 1963 through its 18 cm transitions.[30] In the subsequent years •OH was observed by its rotational transitions at far infrared wavelengths, mainly in the Orion region. Because each rotational level of •OH is split in by lambda doubling, astronomers can observe a wide variety of energy states from the ground state.
Tracer of shock conditions
Very high densities are required to thermalize the rotational transitions of •OH,[31] so it is difficult to detect far-infrared emission lines from a quiescent molecular cloud. Even at H2 densities of 106 cm−3, dust must be optically thick at infrared wavelengths. But the passage of a shock wave through a molecular cloud is precisely the process which can bring the molecular gas out of equilibrium with the dust, making observations of far-infrared emission lines possible. A moderately fast shock may produce a transient raise in the •OH abundance relative to hydrogen. So, it is possible that far-infrared emission lines of •OH can be a good diagnostic of shock conditions.
In diffuse clouds
Diffuse clouds are of astronomical interest because they play a primary role in the evolution and thermodynamics of ISM. Observation of the abundant atomic hydrogen in 21 cm has shown good signal-to-noise ratio in both emission and absorption. Nevertheless, HI observations have a fundamental difficulty when they are directed at low mass regions of the hydrogen nucleus, as the center part of a diffuse cloud: the thermal width of the hydrogen lines are of the same order as the internal velocities of structures of interest, so cloud components of various temperatures and central velocities are indistinguishable in the spectrum. Molecular line observations in principle do not suffer from this problem. Unlike HI, molecules generally have excitation temperature Tex ≪ Tkin, so that emission is very weak even from abundant species. CO and •OH are the most easily studied candidate molecules. CO has transitions in a region of the spectrum (wavelength < 3 mm) where there are not strong background continuum sources, but •OH has the 18 cm emission, line convenient for absorption observations.[32] Observation studies provide the most sensitive means of detections of molecules with subthermal excitation, and can give the opacity of the spectral line, which is a central issue to model the molecular region.
Studies based in the kinematic comparison of •OH and H I absorption lines from diffuse clouds are useful in determining their physical conditions, especially because heavier elements provide higher velocity resolution.
Masers
•OH masers, a type of astrophysical maser, were the first masers to be discovered in space and have been observed in more environments than any other type of maser.
In the Milky Way, •OH masers are found in stellar masers (evolved stars), interstellar masers (regions of massive star formation), or in the interface between supernova remnants and molecular material. Interstellar •OH masers are often observed from molecular material surrounding ultracompact H II regions (UC H II). But there are masers associated with very young stars that have yet to create UC H II regions.[33] This class of •OH masers appears to form near the edges of very dense material, place where H2O masers form, and where total densities drop rapidly and UV radiation form young stars can dissociate the H2O molecules. So, observations of •OH masers in these regions, can be an important way to probe the distribution of the important H2O molecule in interstellar shocks at high spatial resolutions.
See also
References
- 1 2 "Hydroxyl (CHEBI:29191)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ↑ Hayyan, M.; Hashim, M.A.; AlNashef, I.M. (2016). "Superoxide Ion: Generation and Chemical Implications". Chem. Rev. 116 (5): 3029–3085. doi:10.1021/acs.chemrev.5b00407. PMID 26875845.
- ↑ McNaught, A. D.; Wilkinson, A. (2014). "radical (free radical)". IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Blackwell Scientific Publications, Oxford. doi:10.1351/goldbook.R05066. Retrieved 12 April 2020.
- ↑ "Experimental data for OH (Hydroxyl radical)". Computational Chemistry Comparison and Benchmark DataBase.
- ↑ Maeda, Kenji; Wall, Michael L.; Carr, Lincoln D. (2015). "Hyperfine structure of the hydroxyl free radical (OH) in electric and magnetic fields". New Journal of Physics. 17 (4): 045014. arXiv:1410.3849. Bibcode:2015NJPh...17d5014M. doi:10.1088/1367-2630/17/4/045014. S2CID 101652013.
- 1 2 Xu, Guozhong; Chance, Mark R. (2007). "Hydroxyl Radical-Mediated Modification of Proteins as Probes for Structural Proteomics". Chemical Reviews. 107 (8): 3514–3543. doi:10.1021/cr0682047. PMID 17683160.
- ↑ Halliwell, Barry; Adhikary, Amitava; Dingfelder, Michael; Dizdaroglu, Miral (2021). "Hydroxyl radical is a significant player in oxidative DNA damage in vivo". Chemical Society Reviews. 50 (15): 8355–8360. doi:10.1039/d1cs00044f. PMC 8328964. PMID 34128512.
- ↑ Sunil Paul, M. M.; Aravind, Usha K.; Pramod, G.; Aravindakumar, C.T. (April 2013). "Oxidative degradation of fensulfothion by hydroxyl radical in aqueous medium". Chemosphere. 91 (3): 295–301. Bibcode:2013Chmsp..91..295S. doi:10.1016/j.chemosphere.2012.11.033. PMID 23273737.
- ↑ Sreekanth R, Prasanthkumar KP, Sunil Paul MM, Aravind UK, Aravindakumar CT (Nov 7, 2013). "Oxidation reactions of 1- and 2-naphthols: an experimental and theoretical study". The Journal of Physical Chemistry A. 117 (44): 11261–70. Bibcode:2013JPCA..11711261S. doi:10.1021/jp4081355. PMID 24093754.
- ↑ Gligorovski, Sasho; Strekowski, Rafal; Barbati, Stephane; Vione, Davide (2015). "Environmental Implications of Hydroxyl Radicals (•OH)". Chemical Reviews. 115 (24): 13051–13092. doi:10.1021/cr500310b. PMID 26630000.
- ↑ Isaksen, I.S.A.; S.B. Dalsøren (2011). "Getting a better estimate of an atmospheric radical". Science. 331 (6013): 38–39. Bibcode:2011Sci...331...38I. doi:10.1126/science.1199773. PMID 21212344. S2CID 206530807.
- ↑ "Trends in the Hydroxyl Free Radical" (PDF) (IPCC AR4 WG1). IPCC.
The hydroxyl free radical (OH) is the major oxidizing chemical in the atmosphere, destroying about 3.7 billion tonnes of trace gases, including methane and all HFCs and HCFCs, each year (Ehhalt, 1999).
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Heal MR, Heard DE, Pilling MJ, Whitaker BJ (1995). "On the development and validation of FAGE for local measurement of tropospheric OH and HO2" (PDF). Journal of the Atmospheric Sciences. 52 (19): 3428–3448. Bibcode:1995JAtS...52.3428H. doi:10.1175/1520-0469(1995)052<3428:OTDAVO>2.0.CO;2. ISSN 1520-0469.
- ↑ Montzka, S.A.; M. Krol; E. Dlugokencky; B. Hall; P. Jöckel; J. Lelieveld (2011). "Small interannual variability of global atmospheric hydroxyl". Science. 331 (6013): 67–69. Bibcode:2011Sci...331...67M. doi:10.1126/science.1197640. PMID 21212353. S2CID 11001130. Retrieved 2011-01-09.
- ↑ ["Like a giant elevator to the stratosphere", News Release, Alfred Wegener Institute, April 3, 2014]
- ↑ Hartquist, T. W., ed. (1990-04-12). Molecular Astrophysics: A volume honouring Alexander Dalgarno. Cambridge England ; New York: Cambridge University Press. p. 500. ISBN 978-0-521-36331-0.
- ↑ Weinreb et al., Nature, Vol. 200, pp. 829, 1963
- ↑ Dieter, N. H.; Ewen, H. I. (1964). "Radio Observations of the Interstellar OH Line at 1,667 Mc/s". Nature. 201 (4916): 279–281. Bibcode:1964Natur.201..279D. doi:10.1038/201279b0. ISSN 0028-0836. S2CID 4163406.
- ↑ Robinson, B J; McGee, R X (1967). "OH Molecules in the Interstellar Medium". Annual Review of Astronomy and Astrophysics. 5 (1): 183–212. Bibcode:1967ARA&A...5..183R. doi:10.1146/annurev.aa.05.090167.001151. ISSN 0066-4146.
- ↑ Heiles, Carl E. (1968). "Normal OH Emission and Interstellar Dust Clouds". The Astrophysical Journal. 151: 919. Bibcode:1968ApJ...151..919H. doi:10.1086/149493. ISSN 0004-637X.
- ↑ Rank, D. M.; Townes, C. H.; Welch, W. J. (1971). "Interstellar Molecules and Dense Clouds". Science. 174 (4014): 1083–1101. Bibcode:1971Sci...174.1083R. doi:10.1126/science.174.4014.1083. ISSN 0036-8075. PMID 17779392. S2CID 43499656.
- ↑ Baud, B.; Wouterloot, J. G. A. (1980), "OH observations of molecular complexes in Orion and Taurus", Astronomy and Astrophysics, 90: 297, Bibcode:1980A&A....90..297B
- ↑ Crutcher, R. M.; Troland, T. H.; Heiles, C. (1981). "Magnetic fields in molecular clouds - OH Zeeman observations". The Astrophysical Journal. 249: 134. Bibcode:1981ApJ...249..134C. doi:10.1086/159268. ISSN 0004-637X.
- ↑ Storey, J. W. V.; Watson, D. M.; Townes, C. H. (1981). "Detection of interstellar OH in the far-infrared". The Astrophysical Journal. 244: L27. Bibcode:1981ApJ...244L..27S. doi:10.1086/183472. ISSN 0004-637X.
- ↑ Baan, Willem A.; Haschick, Aubrey D.; Henkel, Christian (1989). "Molecular outflows in powerful OH megamasers". The Astrophysical Journal. 346: 680. Bibcode:1989ApJ...346..680B. doi:10.1086/168050. ISSN 0004-637X.
- 1 2 Harju, J.; Winnberg, A.; Wouterloot, J. G. A. (2000), "The distribution of OH in Taurus Molecular Cloud-1", Astronomy and Astrophysics, 353: 1065, Bibcode:2000A&A...353.1065H
- ↑ Field, D.; Adams, N. G.; Smith, D. (1980). "Molecular synthesis in interstellar clouds: the radiative association reaction H + OH → H2O + hν". Monthly Notices of the Royal Astronomical Society. 192: 1–10. Bibcode:1980MNRAS.192....1F. doi:10.1093/mnras/192.1.1.
- ↑ "The UMIST Database for Astrochemistry 2012 / astrochemistry.net".
- ↑ Rank, D. M.; Townes, C. H.; Welch, W. J. (1971-12-01). "Interstellar Molecules and Dense Clouds". Science. 174 (4014): 1083–1101. Bibcode:1971Sci...174.1083R. doi:10.1126/science.174.4014.1083. PMID 17779392. S2CID 43499656.
- ↑ Dieter, N. H.; Ewen, H. I. (1964-01-18). "Radio Observations of the Interstellar HO Line at 1,667 Mc/s". Nature. 201 (4916): 279–281. Bibcode:1964Natur.201..279D. doi:10.1038/201279b0. S2CID 4163406.
- ↑ Storey, J. W. V.; Watson, D. M.; Townes, C. H. (1981-02-15). "Detection of interstellar HO in the far-infrared". Astrophysical Journal Letters. 244: L27–L30. Bibcode:1981ApJ...244L..27S. doi:10.1086/183472.
- ↑ Dickey, J. M.; Crovisier, J.; Kazes, I. (May 1981). "Emission-absorption observations of •HO in diffuse interstellar clouds". Astronomy and Astrophysics. 98 (2): 271–285. Bibcode:1981A&A....98..271D.
- ↑ Argon, Alice L.; Reid, Mark J.; Menten, Karl M. (August 2003). "A class of interstellar •HO masers associated with protostellar outflows". The Astrophysical Journal. 593 (2): 925–930. arXiv:astro-ph/0304565. Bibcode:2003ApJ...593..925A. doi:10.1086/376592. S2CID 16367529.
- Downes A.; Blunt T.P. (1879). "The effect of sunlight upon hydrogen peroxide". Nature. 20 (517): 521. Bibcode:1879Natur..20Q.521.. doi:10.1038/020521a0.


