 | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium molybdate | |
| Other names
Disodium molybdate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.683 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Na2MoO4 | |
| Molar mass | 205.92 g/mol (anhydrous) 241.95 g/mol (dihydrate) |
| Appearance | White powder |
| Density | 3.78 g/cm3, solid |
| Melting point | 687 °C (1,269 °F; 960 K) |
| 84 g/100 ml (100 °C) | |
Refractive index (nD) |
1.714 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4000 mg/kg (rat, oral)[1] |
LC50 (median concentration) |
>2080 mg/m3 (rat, 4 hr)[1] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions |
Sodium chromate Sodium tungstate |
Other cations |
Ammonium molybdate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium molybdate, Na2MoO4, is useful as a source of molybdenum.[2] This white, crystalline salt is often encountered as the dihydrate, Na2MoO4·2H2O.
Preparation
Dissolution of MoO3 in sodium hydroxide at 50–70 °C followed by crystallizing the filtered product.[3] If crystallized below 10 °C, the decahydrate forms. Above 10 °C, the dihydate crystallizes. The anhydrous salt is obtained by heating this product at 100 °C.
- MoO3 + 2NaOH + H2O → Na2MoO4·2H2O
Uses
The agriculture industry uses 1 million pounds per year as a fertilizer. In particular, its use has been suggested for treatment of whiptail in broccoli and cauliflower in molybdenum-deficient soils.[4][5] However, care must be taken because at a level of 0.3 ppm sodium molybdate can cause copper deficiencies in animals, particularly cattle.[3]
It is used in industry for corrosion inhibition, as it is a non-oxidizing anodic inhibitor.[3] The addition of sodium molybdate significantly reduces the nitrite requirement of fluids inhibited with nitrite-amine, and improves the corrosion protection of carboxylate salt fluids.[6] In industrial water treatment applications where galvanic corrosion is a potential due to bimetallic construction, the application of sodium molybdate is preferred over sodium nitrite. Sodium molybdate has the advantage in that the dosing of lower ppm's of molybdate allow for lower conductivity of the circulating water. Sodium molybdate at levels of 50-100 ppm offer the same levels of corrosion inhibition as sodium nitrite at levels of 800+ ppm. By utilizing lower concentrations of sodium molybdate, conductivity is kept at a minimum and thus galvanic corrosion potentials are decreased.[7]
Reactions
When treated with sodium borohydride, molybdate is reduced to molybdenum(IV) oxide:[8]
- Na2MoO4 + NaBH4 + 2H2O → NaBO2 + MoO2 + 2NaOH + 3H2
Sodium molybdate reacts with the acids of dithiophosphates:[3]
- Na2MoO4 + → [MoO2(S2P(OR)2)2]
which further reacts to form [MoO3(S2P(OR)2)4].
Structure
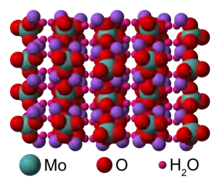
In aqueous solution, sodium molybdate features dissociated sodium ions and tetrahedral molybdate (MoO42-), which adopts a sulfate-like structure. The solid dihydrate material has a complex structure typical for alkali metal salts of oxyanions. The MoO42- subunits are tetrahedral with Mo-O distances near 178 pm.[3][9]
Safety
Sodium molybdate supports the biosynthesis of molybdoenzymes, which are found in all higher forms of life.[10] The LC50 for freshwater fish ranges from 60 to 7630 mg/L. The toxicity of soluble molybdate to marine organisms has also been reported.[11]
References
- 1 2 "Molybdenum (soluble compounds, as Mo)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
- 1 2 3 4 5 Braithwaite, E.R.; Haber, J. Molybdenum: An outline of its Chemistry and Uses. 1994. Elsevier Science B.V. Amsterdam, the Netherlands.
- ↑ Plant, W. (1950). "Use of Lime and Sodium Molybdate for the Control of 'Whiptail' in Broccoli". Nature. 165 (4196): 533. Bibcode:1950Natur.165..533P. doi:10.1038/165533b0. S2CID 4213274.
- ↑ Davies, E. B. (1945). "A Case of Molybdenum Deficiency in New Zealand". Nature. 156 (3961): 392. Bibcode:1945Natur.156..392D. doi:10.1038/156392b0. S2CID 4071159.
- ↑ Vukasovich, Mark S. Lubrication Engineering 1980. 36(12). 708-12.
- ↑ M. Houser, Corrosion Control Services, Inc., Introduction Handbook
- ↑ Tsang, Chi Fo; Manthiram, Arumugam (1997). "Synthesis of lower-valent molybdenum oxides in aqueous solutions by reducing Na2MoO4 with NaBH4". Journal of Materials Chemistry. 7 (6): 1003–1006. doi:10.1039/A606389F. ISSN 1364-5501.
- ↑ Matsumoto, Kazuko; Kobayashi, Akiko; Sasaki, Yukiyoshi (1975). "The Crystal Structure of Sodium Molybdate Dihydrate, Na2MoO4·2H2O". Bulletin of the Chemical Society of Japan. 48 (3): 1009–1013. doi:10.1246/bcsj.48.1009.
- ↑ "Linus Pauling Institute page on molybdenum". 23 April 2014.
- ↑ Heijerick, D.G.; Regoli, L.; Stubblefield, W. (2012). "The chronic toxicity of molybdate to marine organisms. I. Generating reliable effects data". Science of the Total Environment. 430: 260–269. Bibcode:2012ScTEn.430..260H. doi:10.1016/j.scitotenv.2012.03.045. PMID 22663766.
