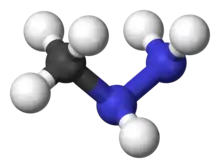
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methylhydrazine[1] | |
| Other names
Methyldiazane, monomethyl hydrazine | |
| Identifiers | |
3D model (JSmol) |
|
| 635645 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.429 |
| EC Number |
|
| MeSH | Monomethylhydrazine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1244 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH6N2 | |
| Molar mass | 46.073 g·mol−1 |
| Appearance | Fuming, colourless liquid |
| Odor | Fish-like [2] |
| Density | 875 mg/mL (at 20 °C) |
| Melting point | −52 °C (−62 °F; 221 K) |
| Boiling point | 87.50 °C; 189.50 °F; 360.65 K |
| Miscible[3] | |
| log P | −1.318 |
| Vapor pressure | 5.00 kPa (at 20 °C) |
Refractive index (nD) |
1.4325 |
| Thermochemistry | |
Heat capacity (C) |
134.93 J/(K·mol) |
Std molar entropy (S⦵298) |
165.94 J/(K·mol) |
Std enthalpy of formation (ΔfH⦵298) |
54.14 kJ/mol |
Std enthalpy of combustion (ΔcH⦵298) |
−1305.8 to −1304.6 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
highly toxic and reactive liquid |
| GHS labelling: | |
  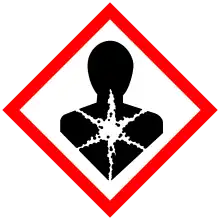 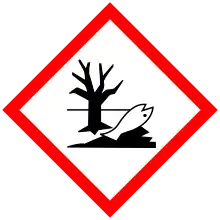 | |
| Danger | |
| H225, H300, H301, H311, H314, H330, H351, H411 | |
| P210, P260, P273, P280, P284 | |
| NFPA 704 (fire diamond) | |
| Flash point | −8 °C; 17 °F; 265 K[3] |
| 196 °C (385 °F; 469 K) | |
| Explosive limits | 2.5–92%[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
32 mg/kg (oral, rat) |
LC50 (median concentration) |
|
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
C 0.2 ppm (0.35 mg/m3) [skin][3] |
REL (Recommended) |
Ca C 0.04 ppm (0.08 mg/m3) [2-hr][3] |
IDLH (Immediate danger) |
Ca [20 ppm][3] |
| Safety data sheet (SDS) | inchem.org |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Monomethylhydrazine (mono-methyl hydrazine, MMH) is a highly toxic, volatile hydrazine derivative with the chemical formula CH6N2. It is used as a rocket propellant in bipropellant rocket engines because it is hypergolic with various oxidizers such as nitrogen tetroxide (N2O4) and nitric acid (HNO3). As a propellant, it is described in specification MIL-PRF-27404.[5]
MMH is a hydrazine derivative that was once used in the orbital maneuvering system (OMS) and reaction control system (RCS) engines of NASA's Space Shuttle, which used MMH and MON-3 (a mixture of nitrogen tetroxide with approximately 3% nitric oxide). This chemical is toxic and carcinogenic,[6] but it is easily stored in orbit, providing moderate performance for very low fuel tank system weight. MMH and its chemical relative unsymmetrical dimethylhydrazine (UDMH) have a key advantage that they are stable enough to be used in regeneratively cooled rocket engines. The European Space Agency (ESA) has attempted to seek new options in terms of bipropellant rocket combinations to avoid using deadly chemicals such as MMH and its relatives.[7]
MMH is believed to be the main cause of the toxicity of mushrooms of genus Gyromitra, especially the false morel (Gyromitra esculenta). In these cases, MMH is formed by the hydrolysis of gyromitrin.[8]
Monomethylhydrazine is considered to be a possible occupational carcinogen,[9] and the occupational exposure limits to MMH are set at protective levels to account for the possible carcinogenicity.[10]
A known use of MMH is in the synthesis of suritozole.
MMH is also assumed to be the active methylating agent in the drug Temozolomide.[11]
References
- ↑ "Monomethylhydrazine - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 2 May 2012.
- ↑ Methylhydrazine: odor
- 1 2 3 4 5 6 NIOSH Pocket Guide to Chemical Hazards. "#0419". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Methylhydrazine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ MIL-PRF-27404 (1997-10-01). "Performance Specification, Propellant, Monomethylhydrazine". Archived from the original on 2012-03-20. Retrieved 2011-05-21.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ↑ Monomethylhydrazine as toxic and carcinogenic chemical substance
- ↑ Preliminary Programme. International Conference on Green Propellant for Space Propulsion. Noordwijk, NL: European Space Agency. 20–22 June 2001.
- ↑ Pyysalo, H. (1975). "Some new toxic compounds in false morels, Gyromitra esculenta". Naturwissenschaften. 62 (8): 395. Bibcode:1975NW.....62..395P. doi:10.1007/BF00625355. PMID 1238907. S2CID 178876.
- ↑ Immediately Dangerous to Life or Health Concentrations (IDLHs) (Report). U.S. Centers for Disease Control and Prevention.
- ↑ NIOSH Pocket Guide to Chemical Hazards (Report). U.S. Centers for Disease Control and Prevention. NPGD #0419.
- ↑ "Google Scholar". scholar.google.com. Retrieved 2022-04-17.
External links
 Media related to Monomethylhydrazine at Wikimedia Commons
Media related to Monomethylhydrazine at Wikimedia Commons
