| Clinical data | |
|---|---|
| Trade names | TNKase |
| AHFS/Drugs.com | Monograph |
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Liver |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C2561H3919N747O781S40 |
| Molar mass | 58951.37 g·mol−1 |
| | |
Tenecteplase, sold under the trade names TNKase, Metalyse and Elaxim, is an enzyme used as a thrombolytic drug.
Tenecteplase is a tissue plasminogen activator (tPA) produced by recombinant DNA technology using an established mammalian cell line (Chinese hamster ovary cells). Tenecteplase is a 527 amino acid glycoprotein developed by introducing the following modifications to the complementary DNA for natural human tPA: a substitution of threonine 103 with asparagine, and a substitution of asparagine 117 with glutamine, both within the kringle 1 domain, and a tetra-alanine substitution at amino acids 296–299 in the protease domain.
Tenecteplase is a recombinant fibrin-specific plasminogen activator that is derived from native t-PA by modifications at three sites of the protein structure. It binds to the fibrin component of the thrombus (blood clot) and selectively converts thrombus-bound plasminogen to plasmin, which degrades the fibrin matrix of the thrombus. Tenecteplase has a higher fibrin specificity and greater resistance to inactivation by its endogenous inhibitor (PAI-1) compared to native t-PA.
The abbreviation TNK is common for referring to tenecteplase, but abbreviating drug names is not best practice in medicine, and in fact "TNK" is one of the examples given on the Institute for Safe Medication Practices do-not-use list.
Research
Researchers at Newcastle University in Australia say they have had a significant breakthrough in treating stroke patients using the commonly used drug.[1] The findings were published in the New England Medical Journal. Though safety has been established through previous clinical trials, there is ongoing debate about whether this is an effective treatment for ischemic stroke, and significant ongoing discussion between emergency physicians, neurologists and pharmacists about whether this treatment should be used for that indication.
The American Heart Association/American Stroke Association 2019 update to the 2018 guidelines for the Early Management of Acute Ischemic Stroke supports considering tenecteplase over alteplase in patients without contraindication to intravenous thrombolytics.[2]
Pharmacokinetics
Distribution: approximates plasma volume
Metabolism: Primarily hepatic
Half-life elimination: Biphasic: Initial: 20–24 minutes; Terminal: 90–130 minutes
Excretion: Clearance: Plasma: 99-119 mL/minute
Gallery
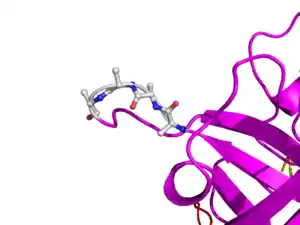
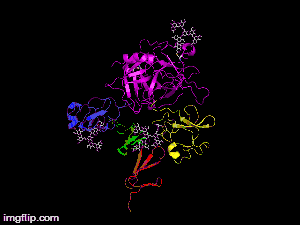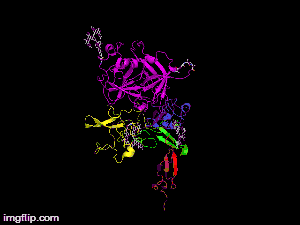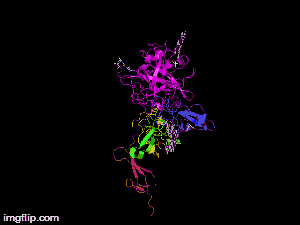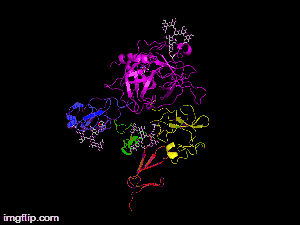 Here is TNK-tPA. It is very similar to t-PA, but the glycosylation occurring in Kringle 1 is manipulated. The mutation T103N means that glycosylation occurs at that point. The mutation N117Q means that the high mannose sugar residue is absent at that point
Here is TNK-tPA. It is very similar to t-PA, but the glycosylation occurring in Kringle 1 is manipulated. The mutation T103N means that glycosylation occurs at that point. The mutation N117Q means that the high mannose sugar residue is absent at that point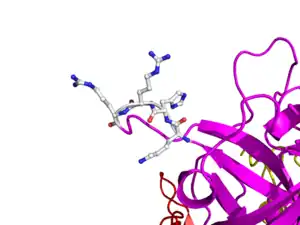
References
- ↑ Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. (March 2012). "A randomized trial of tenecteplase versus alteplase for acute ischemic stroke". The New England Journal of Medicine. 366 (12): 1099–1107. doi:10.1056/NEJMoa1109842. hdl:1959.13/1039697. PMID 22435369.
- ↑ Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. (December 2019). "Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke. 50 (12): e344–e418. doi:10.1161/str.0000000000000211. PMID 31662037. S2CID 204973899.
Further reading
- Gurbel PA, Hayes K, Bliden KP, Yoho J, Tantry US (January 2005). "The platelet-related effects of tenecteplase versus alteplase versus reteplase". Blood Coagulation & Fibrinolysis. 16 (1): 1–7. doi:10.1097/00001721-200501000-00001. PMID 15650539. S2CID 44664652.
- Melzer C, Richter C, Rogalla P, Borges AC, Theres H, Baumann G, Laule M (August 2004). "Tenecteplase for the treatment of massive and submassive pulmonary embolism". Journal of Thrombosis and Thrombolysis. 18 (1): 47–50. doi:10.1007/s11239-004-0174-z. PMID 15744554. S2CID 10947258.
- Ohman EM, Van de Werf F, Antman EM, Califf RM, de Lemos JA, Gibson CM, et al. (July 2005). "Tenecteplase and tirofiban in ST-segment elevation acute myocardial infarction: results of a randomized trial". American Heart Journal. 150 (1): 79–88. doi:10.1016/j.ahj.2005.01.007. PMID 16084152.
- De Luca G, Suryapranata H, Chiariello M (December 2005). "Tenecteplase followed by immediate angioplasty is more effective than tenecteplase alone for people with STEMI. Commentary". Evidence-Based Cardiovascular Medicine. 9 (4): 284–287. doi:10.1016/j.ebcm.2005.09.021. PMID 16380055.
- "Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial". Lancet. 367 (9510): 569–578. February 2006. doi:10.1016/S0140-6736(06)68147-6. PMID 16488800. S2CID 23972378.
- Bozeman WP, Kleiner DM, Ferguson KL (June 2006). "Empiric tenecteplase is associated with increased return of spontaneous circulation and short term survival in cardiac arrest patients unresponsive to standard interventions". Resuscitation. 69 (3): 399–406. doi:10.1016/j.resuscitation.2005.09.027. PMID 16563599.
- Hull JE, Hull MK, Urso JA, Park HA (April 2006). "Tenecteplase in acute lower-leg ischemia: efficacy, dose, and adverse events". Journal of Vascular and Interventional Radiology. 17 (4): 629–636. doi:10.1097/01.RVI.0000202751.74625.79. PMID 16614145.
- Peacock M (22 March 2012). "Stroke patients make 'Lazarus-like' recovery". The World Today.
- Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. (March 2012). "A randomized trial of tenecteplase versus alteplase for acute ischemic stroke". The New England Journal of Medicine. 366 (12): 1099–1107. doi:10.1056/NEJMoa1109842. hdl:1959.13/1039697. PMID 22435369.