 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Benzenehexacarboxylic acid | |||
| Other names
Graphitic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.007.495 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C12H6O12 | |||
| Molar mass | 342.16 g/mol | ||
| Density | 1.68 g/cm3, 2.078 (calc.)[3] | ||
| Melting point | > 300 °C (572 °F; 573 K) | ||
| Boiling point | 678 °C (1,252 °F; 951 K) (calc.)[3] | ||
| Acidity (pKa) | 5.0, 2.19, 3.31, 4.78, 5.89, 6.96[4] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
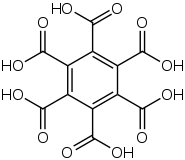
Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid.[5] It crystallizes in fine silky needles and is soluble in water and alcohol.
Structure
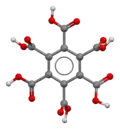
The stable conformation of this molecule has the carboxylic acid groups rotated out of the plane of the central benzene ring. The molecule adopts a propeller-like conformation in which the tilt of each carboxylic acid group relative to the central benzene ring varies due to intramolecular hydrogen bonding.[2]
Preparation
Mellitic acid may be prepared by warming mellite with ammonium carbonate, boiling off the excess of the ammonium salt, and adding ammonia to the solution. The precipitated alumina is filtered off, the filtrate evaporated, and the ammonium salt of the acid purified by recrystallization. The ammonium salt is then converted into the lead salt by precipitation with lead acetate, and the lead salt is then decomposed by hydrogen sulfide. The acid may also be prepared by the oxidation of pure carbon, graphite or hexamethylbenzene, by alkaline potassium permanganate in the cold, or by hot concentrated nitric acid.[6]
Reactions
It is a very stable compound; chlorine, concentrated nitric acid and hydriodic acid do not react with it. It is decomposed, on dry distillation, into carbon dioxide and pyromellitic acid, C10H6O8; when distilled with lime it gives carbon dioxide and benzene. Long digestion of the acid with an excess of phosphorus pentachloride forms the acid chloride, which crystallizes in needles, and melts at 190 °C. By heating the ammonium salt of the acid to 150–160 °C while ammonia is evolved, a mixture of paramide (mellimide, molecular formula C
6(CONHCO)
3), and ammonium euchroate is obtained. The mixture may be separated by dissolving out the ammonium euchroate with water. Paramide is a white amorphous powder, insoluble in water and alcohol.
The high stability of mellitic acid salts and their presence as an endproduct of the oxidation of polycyclic aromatic hydrocarbons, which are present in the solar system, make them a possible organic substance in Martian soil.[7]
Mellitates (and salts of other benzene polycarboxylic acids) of iron and cobalt have interesting magnetic properties.[8]
See also
References
- ↑ MSDS for mellitic acid
- ↑ Bart, J. C. J. (1968). "The crystal structure of a modification of hexaphenylbenzene". Acta Crystallographica Section B. 24 (10): 1277–1287. doi:10.1107/S0567740868004176.
- 1 2 Curate Data: Predicted Properties: 2244. ChemSpider.com.
- ↑ Brown, H.C., et al., in Baude, E.A. and Nachod, F.C., Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955.
- ↑ Klaproth (1802). Beiträge zur chemischen Kenntniss der Mineralkörper, Band 3 (in German). p. 114.
- ↑ WebElements.com
- ↑ S. A. Benner; K. G. Devine; L. N. Matveeva; D. H. Powell (2000). "The missing organic molecules on Mars". Proceedings of the National Academy of Sciences. 97 (6): 2425–2430. doi:10.1073/pnas.040539497. PMC 15945. PMID 10706606.
- ↑ Kurmoo M, Estournes C, Oka Y, Kumagai H, Inoue K (2005) Inorganic Chemistry volume 44, page 217
Further reading
Henry Enfield Roscoe, Carl Scholemmer, "Mellitene Group", "A Treatise on Chemistry: V.III: The Chemistry of the Hydrocarbons and their Derivatives on Organic Chemistry: P.V:529. D. Appleton and Co. (1889).
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Mellitic Acid". Encyclopædia Britannica. Vol. 18 (11th ed.). Cambridge University Press. p. 95.