 | |
| Names | |
|---|---|
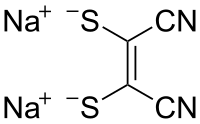
| Preferred IUPAC name
Disodium (Z)-1,2-dicyanoethene-1,2-bis(thiolate) | |
| Other names
Sodium mnt sodium maleonitriledithiolate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C4N2Na2S2 | |
| Molar mass | 186.16 g·mol−1 |
| Appearance | yellow solid |
| Solubility in ethanol, DMF | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium maleonitriledithiolate is the chemical compound described by the formula Na2S2C2(CN)2. The name refers to the cis compound, structurally related to maleonitrile ((CH(CN))2). Maleonitriledithiolate is often abbreviated mnt. It is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate. It is a prototypical non-innocent ligand in coordination chemistry. Several complexes are known, such as [Ni(mnt)2]2−.[2]: 143–146
2Ni(mnt)2.jpg.webp)
Sample of ([(CH3CH2)4N]+)2[Ni(mnt)2]2−.
The salt is synthesized by treating carbon disulfide with sodium cyanide to give the cyanodithioformate salt, which eliminates elemental sulfur in aqueous solution:[3]
- 8 NaCN + 8 CS2 → 4 Na2S2C2(CN)2 + S8
The compound was first described by Bähr and Schleitzer 1958.[4]
References
- ↑ Chem Sources U.S.A. Directories Publishing Company, Incorporated. 2001. p. 535. ISBN 978-0-937020-34-0.
- ↑ Day, Peter; Coronado, Eugenio (2004-12-14). Miller, Joel S.; Drillon, Marc (eds.). Molecular Materials Combining Magnetic and Conducting Properties (1 ed.). Wiley. pp. 105–159. doi:10.1002/3527604383.ch4. ISBN 978-3-527-30665-7.
- ↑ Davison, A.; Holm, R. H.; Benson, R. E.; Mahler, W. (January 1967). Muetterties, Earl L. (ed.). Metal Complexes Derived from cis ‐1,2‐dicyano‐1,2‐ethylenedithiolate and Bis(Trifluoromethyl)‐1,2‐dithiete. Vol. 10 (1 ed.). Wiley. pp. 8–26. doi:10.1002/9780470132418.ch3. ISBN 978-0-470-13169-5.
- ↑ G. Bähr and G. Schleitzer (1957). "Beiträge zur Chemie des Schwefelkohlenstoffs und Selenkohlenstoffs, II. Die Kondensierende Spontan-Entschwefelung von Salzen und Estern der Cyan-Dithioameisensäure. Freie Cyan-Dithioameisensäure". Chemische Berichte. 90 (3): 438–443. doi:10.1002/cber.19570900322.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.