 | |
| Clinical data | |
|---|---|
| Trade names | Pluvicto |
| Other names | 177Lu-PSMA-617, Lutetium Lu 177 vipivotide tetraxetan (USAN US) |
| License data | |
| Routes of administration | Intravenous |
| Drug class | Radiopharmaceutical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
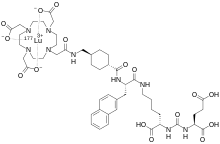
Lutetium (177Lu) vipivotide tetraxetan, sold under the brand name Pluvicto, is a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).[3][4] Lutetium (177Lu) vipivotide tetraxetan is a targeted radioligand therapy.[4][6]
The most common adverse reactions include fatigue, dry mouth, nausea, anemia, decreased appetite, and constipation.[4][5]
Lutetium (177Lu) vipivotide tetraxetan is a radioconjugate composed of PSMA-617, a human prostate-specific membrane antigen (PSMA)-targeting ligand, conjugated to the beta-emitting radioisotope lutetium-177, with potential antineoplastic activity against PSMA-expressing tumor cells.[7] Upon intravenous administration of lutetium (177Lu) vipivotide tetraxetan, vipivotide tetraxetan targets and binds to PSMA-expressing tumor cells.[7] Upon binding, PSMA-expressing tumor cells are destroyed by 177Lu through the specific delivery of beta particle radiation.[7] PSMA, a tumor-associated antigen and type II transmembrane protein, is expressed on the membrane of prostatic epithelial cells and overexpressed on prostate tumor cells.[7]
Lutetium (177Lu) vipivotide tetraxetan was approved for medical use in the United States in March 2022,[4][8] and in the European Union in December 2022.[5] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[9][10]
History
In 2006, scientists from Purdue University designed a targeting ligand that bound with high affinity and specificity to PSMA on prostate cancer cells and patented[11][12] its ability to target attached radionuclides such as 177Lu, 99mTc, 68Ga, etc. to prostate cancers. The patents were licensed to Endocyte in 2007. In 2012, scientists at German Cancer Research Center and University Hospital Heidelberg improved the drug's affinity, patented,[13] and licensed to ABX advanced biomedical compounds, a small German pharmaceutical company, for early clinical development. In 2017, the ABX patent was also acquired by Endocyte[14] and Endocyte together with the above two sets of patents was acquired by Novartis in 2018.[15]
Efficacy and safety was initially investigated as a compassionate access treatment in Germany with high tumor targeting and low doses to normal organs.[16] Physician-scientists from the Peter MacCallum Cancer Centre conducted a phase 2 trial demonstrating high response rates, low toxicity and reduction in pain in men with metastatic castration-resistant cancer who progressed after conventional treatments.[17] The ANZUP co-operative trials conducted the first randomized, multicentre, trial comparing lutetium vipivotide tetraxetan to cabazitaxel chemotherapy.[18] This trial demonstrated higher PSA response and fewer adverse effects with lutetium vipivotide tetraxetan.
Efficacy was evaluated in VISION,[19] a randomized (2:1), multicenter, open-label trial that evaluated lutetium (177Lu) vipivotide tetraxetan plus best standard of care (BSoC) (n=551) or BSoC alone (n=280) in men with progressive, prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC).[4] All participants received a GnRH analog or had prior bilateral orchiectomy.[4] Participants were required to have received at least one androgen receptor pathway inhibitor, and 1 or 2 prior taxane-based chemotherapy regimens.[4] Participants received lutetium (177Lu) vipivotide tetraxetan 7.4 GBq (200 mCi) every 6 weeks for up to a total of 6 doses plus BSoC or BSoC alone.[4]
The U.S. Food and Drug Administration (FDA) granted the application for lutetium (177Lu) vipivotide tetraxetan priority review and breakthrough therapy designations.[4]
Society and culture
Regulatory status
On 13 October 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Pluvicto, intended for the treatment of prostate cancer.[20] The applicant for this medicinal product is Novartis Europharm Limited.[20] Lutetium (177Lu) vipivotide tetraxetan was approved for medical use in the European Union in December 2022.[5][21]
References
- ↑ Advanced Accelerator Applications USA. "Pluvicto Product Monograph" (PDF). The Drug and Health Product Register. Government of Canada. Retrieved 12 October 2022.
- ↑ "Summary Basis of Decision - Pluvicto". Health Canada. 23 October 2014. Retrieved 23 February 2023.
- 1 2 "Pluvicto- lutetium lu 177 vipivotide tetraxetan injection, solution". DailyMed. 23 March 2022. Archived from the original on 5 April 2022. Retrieved 4 April 2022.
- 1 2 3 4 5 6 7 8 9 10 "FDA approves Pluvicto for metastatic castration-resistant prostate can". U.S. Food and Drug Administration. 23 March 2022. Archived from the original on 24 March 2022. Retrieved 23 March 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 "Pluvicto EPAR". European Medicines Agency. 12 October 2022. Retrieved 21 December 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Neels OC, Kopka K, Liolios C, Afshar-Oromieh A (December 2021). "Radiolabeled PSMA Inhibitors". Cancers. 13 (24): 6255. doi:10.3390/cancers13246255. PMC 8699044. PMID 34944875.
- 1 2 3 4 "Lutetium Lu 177 Vipivotide Tetraxetan (Code C148145)". NCI Thesaurus. 28 February 2022. Archived from the original on 15 April 2022. Retrieved 23 March 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Novartis Pluvicto approved by FDA as first targeted radioligand therapy for treatment of progressive, PSMA positive metastatic castration-resistant prostate cancer" (Press release). Novartis. 23 March 2022. Archived from the original on 23 March 2022. Retrieved 23 March 2022.
- ↑ "Advancing Health Through Innovation: New Drug Therapy Approvals 2022". U.S. Food and Drug Administration (FDA). 10 January 2023. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ New Drug Therapy Approvals 2022 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2024. Archived from the original on 14 January 2024. Retrieved 14 January 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ US 11318121, Low PS, Kularatne SA, "PSMA binding ligand-linker conjugates and methods for using", issued 3 May 2022, assigned to Purdue Research Foundation
- ↑ US 10406240, Low PS, Kularatne SA, "PSMA binding ligand-linker conjugates and methods for using", issued 10 September 2019, assigned to Purdue Research Foundation
- ↑ US 20160228587, Eder M, Kopka K, Schäfer M, Bauder-Wüst U, Haberkorn U, Eisenhut M, Mier W , Benesova M, "Labeled inhibitors of prostate specific membrane antigen (psma), their use as imaging agents and pharmaceutical agents for the treatment of prostate cancer", published 11 August 2016, assigned to Deutsches Krebsforschungszentrum DKFZ, Universitaet Heidelberg and Molecular Insight Pharmaceuticals Inc.
- ↑ "Endocyte Announces Exclusive License of Phase 3 Ready PSMA-Targeted Radioligand Therapy for Development in Prostate Cancer". www.isotope.com. Archived from the original on 29 April 2019. Retrieved 13 April 2022.
- ↑ Taylor PN (18 October 2018). "Novartis inks $2.1B Endocyte buyout, furthering radiotherapy push". Fierce Biotech. Archived from the original on 30 November 2020. Retrieved 13 April 2022.
- ↑ Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. (November 2015). "The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions". Journal of Nuclear Medicine. 56 (11): 1697–1705. doi:10.2967/jnumed.115.161299. PMID 26294298. S2CID 23317879.
- ↑ Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. (June 2018). "[177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study". The Lancet. Oncology. 19 (6): 825–833. doi:10.1016/S1470-2045(18)30198-0. PMID 29752180. S2CID 21674458.
- ↑ Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. (February 2021). "[177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial". Lancet. 397 (10276): 797–804. doi:10.1016/s0140-6736(21)00237-3. PMID 33581798. S2CID 231885243.
- ↑ Clinical trial number NCT03511664 for "Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer (VISION)" at ClinicalTrials.gov.
- 1 2 "Pluvicto: Pending EC decision". European Medicines Agency (EMA). 13 October 2022. Retrieved 14 October 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Pluvicto Product information". Union Register of medicinal products. Retrieved 3 March 2023.
External links
- "Lutetium lu 177 vipivotide tetraxetan". Drug Information Portal. U.S. National Library of Medicine.