 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpropan-1-ol | |
| Other names
Isobutyl alcohol IBA 2-Methyl-1-propanol 2-Methylpropyl alcohol Isopropylcarbinol | |
| Identifiers | |
3D model (JSmol) |
|
| 1730878 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.044 |
| EC Number |
|
| 49282 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1212 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C4H10O | |
| Molar mass | 74.122 g/mol |
| Appearance | Colorless liquid |
| Odor | sweet, musty[2] |
| Density | 0.802 g/cm3, liquid |
| Melting point | −108 °C (−162 °F; 165 K) |
| Boiling point | 107.89 °C (226.20 °F; 381.04 K) |
| 8.7 mL/100 mL[3] | |
| log P | 0.8 |
| Vapor pressure | 9 mmHg (20°C)[2] |
Refractive index (nD) |
1.3959 |
| Viscosity | 3.95 cP at 20 °C |
| Hazards[1] | |
| GHS labelling: | |
  | |
| Danger | |
| H226, H315, H318, H335, H336 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 28 °C (82 °F; 301 K) |
| 415 °C (779 °F; 688 K) | |
| Explosive limits | 1.7–10.9% |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published) |
3750 mg/kg (rabbit, oral) 2460 mg/kg (rat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 100 ppm (300 mg/m3)[2] |
REL (Recommended) |
TWA 50 ppm (150 mg/m3)[2] |
IDLH (Immediate danger) |
1600 ppm[2] |
| Safety data sheet (SDS) | ICSC 0113 |
| Related compounds | |
Related butanols |
n-Butanol sec-Butanol tert-Butanol |
Related compounds |
Isobutyraldehyde Isobutyric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
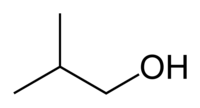
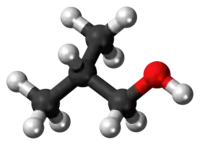
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.[5]
Production
Isobutanol is produced by the carbonylation of propylene. Two methods are practiced industrially, hydroformylation is more common and generates a mixture of isobutyraldehyde and butyraldehyde:
- CH3CH=CH2 + CO + H2 → CH3CH2CH2CHO
The reaction is catalyzed by cobalt or rhodium complexes. The resulting aldehydes are hydrogenated to the alcohols, which are then separated. In Reppe carbonylation, the same products are obtained, but the hydrogenation is effected by the water-gas shift reaction.[5]
Laboratory synthesis
Propanol and methanol can be reacted to produce isobutyl alcohol via Guerbet condensation.[6]
Biosynthesis of isobutanol
E. coli as well as several other organisms has been genetically modified to produce C4 alcohols from glucose, including isobutanol, 1-butanol, 2-methyl-1-butanol, 3-methyl-1-butanol, and 2-phenylethanol. The host's highly active amino acid biosynthetic pathway is shifted to alcohol production. α-Ketoisovalerate, derived from valine, is prone to decarboxylation to give isobutyraldehyde, which is susceptible to reduction to the alcohol:[7]
- (CH3)2CHC(O)CO2H → (CH3)2CHCHO + CO2
- (CH3)2CHCHO + NADH + H+ → (CH3)2CHCH2OH + NAD+
Applications
The uses of isobutanol and n-butanol are similar. They are often used interchangeably. The main applications are as varnishes and precursors to esters, which are useful solvents, e.g. isobutyl acetate. Isobutyl esters of phthalic, adipic, and related dicarboxylic acids are common plasticizers.[5] Isobutanol is also a component of some biofuels.[8]
Safety and regulation
Isobutanol is one of the least toxic of the butanols with an LD50 of 2460 mg/kg (rat, oral).[5]
In March 2009, the Government of Canada announced a ban on isobutanol use in cosmetics.[9]
References
- 1 2 Isobutanol, International Chemical Safety Card 0113, Geneva: International Programme on Chemical Safety, April 2005.
- 1 2 3 4 5 NIOSH Pocket Guide to Chemical Hazards. "#0352". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Iso-butanol". ChemicalLand21.
- ↑ "Isobutyl alcohol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 Hahn, Heinz-Dieter; Dämbkes, Georg; Rupprich, Norbert (2005). "Butanols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_463. ISBN 978-3527306732..
- ↑ Carlini, Carlo; Flego, Cristina; Marchionna, Mario (2004-09-01). "Guerbet condensation of methanol with n-propanol to isobutyl alcohol over heterogeneous copper chromite/Mg–Al mixed oxides catalysts". Molecular Catalysis. 220 (2): 215–220. doi:10.1016/j.molcata.2004.05.034.
The synthesis of isobutyl alcohol from methanol (MeOH) and n-propanol (PrOH) through the Guerbet condensation has been studied [. . .] using catalytic system of copper chromite and Mg-Al mixed oxides.
- ↑ Atsumi, Shota; Hanai, Taizo; Liao, James C. (January 2008). "Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels". Nature. 451 (7174): 86–89. Bibcode:2008Natur.451...86A. doi:10.1038/nature06450. PMID 18172501. S2CID 4413113.
- ↑ Peralta-Yahya, Pamela P.; Zhang, Fuzhong; del Cardayre, Stephen B.; Keasling, Jay D. (15 August 2012). "Microbial engineering for the production of advanced biofuels". Nature. 488 (7411): 320–328. Bibcode:2012Natur.488..320P. doi:10.1038/nature11478. PMID 22895337. S2CID 4423203.
- ↑ "Cosmetic Chemicals Banned in Canada", Chem. Eng. News, 87 (11): 38, 2009-03-16.
External links
- International Chemical Safety Card 0113
- NIOSH Pocket Guide to Chemical Hazards. "#0352". National Institute for Occupational Safety and Health (NIOSH).
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- IPCS Health and Safety Guide 9: Isobutanol
