 | |
| Names | |
|---|---|
| Preferred IUPAC name
Iodosylbenzene[1] | |
| Other names
Iodosobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.864 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5IO | |
| Molar mass | 220.01 g/mol |
| Appearance | colourless solid |
| Density | 1.229 g cm−3 |
| Melting point | 210 ˚C |
| poor | |
| Hazards | |
| GHS labelling:[2] | |
   | |
| Danger | |
| H228, H271, H315, H319, H335 | |
| P210, P220, P240, P241, P261, P264, P264+P265, P271, P280, P283, P302+P352, P304+P340, P305+P351+P338, P306+P360, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P371+P380+P375, P403+P233, P405, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Iodosobenzene or iodosylbenzene is an organoiodine compound with the empirical formula C6H5IO. This colourless solid compound is used as an oxo transfer reagent in research laboratories examining organic and coordination chemistry.
Preparation and structure
Iodosobenzene is prepared from iodobenzene.[3] It is prepared by first oxidizing iodobenzene by peracetic acid. Hydrolysis of resulting diacetate affords "PhIO":[4]
- C6H5I + CH3CO3H + CH3CO2H → C6H5I(O2CCH3)2 + H2O
- C6H5I(O2CCH3)2 + H2O → C6H5IO + 2 CH3CO2H
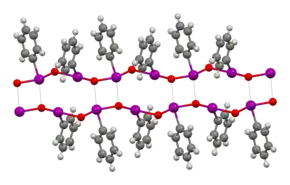
The structure of iodosobenzene has been verified by crystallographically.[5] Related derivatives are also oligomeric.[6] Its low solubility in most solvents and vibrational spectroscopy indicate that it is not molecular, but is polymeric, consisting of –I–O–I–O– chains.[7] The related diacetate, C6H5I(O2CCH3)2, illustrates the ability of iodine(III) to adopt a T-shaped geometry without multiple bonds.[8] Theoretical studies show that the bonding between the iodine and oxygen atoms in iodosobenzene represents a single dative I-O sigma bond, confirming the absence of the double I=O bond.[9]
A monomeric derivative iodosylbenzene is known in the form of 2-(tert-butylsulfonyl)iodosylbenzene, a yellow solid. C-I-O angle is 94.78°, C-I and I-O distances are 2.128 and 1.848 Å.[10]
iodosylbenzene_(MEHKUF).png.webp)
Applications
Iodosobenzene has no commercial uses, but in the laboratory it is employed as an "oxo-transfer reagent." It epoxidizes certain alkenes and converts some metal complexes into the corresponding oxo derivatives. Although it is an oxidant, it is also mildly nucleophilic. These oxo-transfer reactions operate by the intermediacy of adducts PhI=O→M, which release PhI.[11]
A mixture of iodosobenzene and sodium azide in acetic acid converts alkenes to vicinal diazides:.[12][13]
- R2C=CR2 + 2 NaN3 + PhIO + 2 AcOH → (N3)R2C−CR2(N3) + PhI + 2 AcONa + H2O
Safety
This compound is explosive and should not be heated under vacuum.
See also
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 661. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ "Iodosylbenzene". pubchem.ncbi.nlm.nih.gov.
- ↑ Conrad Willgerodt (1892). "Zur Kenntniss aromatischer Jodidchloride, des Jodoso- und Jodobenzols". Ber. 25 (2): 3494–3502. doi:10.1002/cber.189202502221.
- ↑ H. Saltzman, J. G. Sharefkin (1963). "Iodosylbenzene". Organic Syntheses. 43: 60. doi:10.15227/orgsyn.043.0060.
- ↑ Wegeberg, Christina; Frankær, Christian Grundahl; McKenzie, Christine J. (2016). "Reduction of hypervalent iodine by coordination to iron(III) and the crystal structures of PhIO and PhIO2". Dalton Transactions. 45 (44): 17714–17722. doi:10.1039/C6DT02937J. PMID 27761533.
- ↑ Richter, Helen W.; Koser, Gerald F.; Incarvito, Christopher D.; Rheingold, Arnold L. (2007). "Preparation and Structure of a Solid-State Hypervalent-Iodine Polymer Containing Iodine and Oxygen Atoms in Fused 12-Atom Hexagonal Rings". Inorganic Chemistry. 46 (14): 5555–5561. doi:10.1021/ic0701716. PMID 17569525.
- ↑ Hans Siebert; Monika Handrich (1976). "Schwingungsspektren und Struktur von Jodosyl- und Jodyl-Verbindungen". Z. anorg. allg. Chem. 426 (2): 173–183. doi:10.1002/zaac.19764260206.
- ↑ C. J. Carmalt, Claire J.; J. G. Crossley; J. G. Knight; P. Lightfoot; A. Martín; M. P. Muldowney; N. C. Norman; A. G. Orpen (1994). "An examination of the structures of iodosylbenzene (PhIO) and the related imido compound, PhINSO2-4-Me-C6H4, by X-ray powder diffraction and EXAFS (extended X-ray absorption fine structure) spectroscopy". J. Chem. Soc., Chem. Commun. (20): 2367–2368. doi:10.1039/C39940002367.
- ↑ Ivanov, A.; Popov, A.; Boldyrev, A.; Zhdankin, V. (2014). "The I=X (X = O,N,C) Double Bond in Hypervalent Iodine Compounds: Is it Real?". Angew. Chem. Int. Ed. 53 (36): 9617–9621. doi:10.1002/anie.201405142. PMID 25045143.
- ↑ MacIkenas, Dainius; Skrzypczak-Jankun, Ewa; Protasiewicz, John D. (2000). "Redirecting Secondary Bonds to Control Molecular and Crystal Properties of an Iodosyl- and an Iodylbenzene". Angewandte Chemie International Edition. 39 (11): 2007–2010. doi:10.1002/1521-3773(20000602)39:11<2007::AID-ANIE2007>3.0.CO;2-Z. PMID 10941012.
- ↑ Lennartson, Anders; McKenzie, Christine J. (2012). "An Iron(III) Iodosylbenzene Complex: A Masked Non-Heme FeVO". Angewandte Chemie International Edition. 51 (27): 6767–6770. doi:10.1002/anie.201202487. PMID 22639404.
- ↑ Robert M.Moriarty; Jaffar S.Khosrowshahi (1986). "A versatile synthesis of vicinal diazides using hypervalent iodine". Tetrahedron Lett. 27 (25): 2809–2812. doi:10.1016/S0040-4039(00)84648-1.
- ↑ March, J.; Smith, M. B. (2007). Advanced Organic Chemistry (6th ed.). New York: John Wiley & Sons. p. 1182. ISBN 978-0-471-72091-1.