 | |
 | |
| Names | |
|---|---|
| IUPAC name
Iodine trifluoride | |
| Other names
iodine(III) fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| IF3 | |
| Molar mass | 183.9 g/mol |
| Appearance | yellow solid |
| Melting point | decomposes above −28 °C |
| Related compounds | |
Other anions |
iodine trichloride |
Other cations |
bromine trifluoride |
Related compounds |
chlorine trifluoride iodine pentafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Iodine trifluoride is an interhalogen compound with the chemical formula IF3. It is a yellow solid which decomposes above −28 °C. It can be synthesised from the elements, but care must be taken to avoid the formation of IF5.
Reactions
F2 reacts with I2 to yield IF3 at −45 °C in CCl3F. Alternatively, at low temperatures, the fluorination reaction I2 + 3XeF2 → 2IF3 + 3Xe can be used. Not much is known about iodine trifluoride as it is so unstable.
Structure
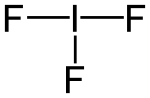
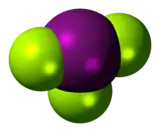
The iodine atom of iodine trifluoride has five electron pairs, of which two are lone-pairs, and the molecule is T-shaped as predicted by VSEPR Theory.[1]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.