 | |
 | |
| Names | |
|---|---|
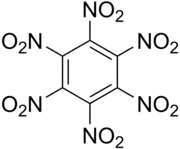
| Preferred IUPAC name
Hexanitrobenzene | |
| Other names
1,2,3,4,5,6-Hexanitrobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6N6O12 | |
| Molar mass | 348.10 g/mol |
| Appearance | Yellow or brown powdered crystals |
| Density | 1.985 g/cm3 |
| Melting point | 256 to 264 °C (493 to 507 °F; 529 to 537 K) |
| Explosive data | |
| Shock sensitivity | None |
| Friction sensitivity | None |
| Detonation velocity | 9,340 m/s[1] |
| RE factor | 1.8 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hexanitrobenzene, also known as HNB, is a nitrobenzene compound in which six nitro groups are bonded to all six positions of a central benzene ring. is a high-density explosive compound with chemical formula C6N6O12, obtained by oxidizing the amine group of pentanitroaniline with hydrogen peroxide in sulfuric acid.
Properties

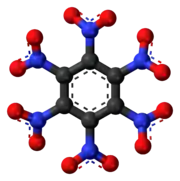
Left: Ball-and-stick structure.
Right: van der Waals space-filling structure.
The stable conformation of this molecule has the nitro groups rotated out of the plane of the central benzene ring. The molecule adopts a propeller-like conformation in which the nitro groups are rotated about 53° from planar.[2]
HNB has the undesirable property of being moderately sensitive to light and, therefore, hard to utilize safely. As of 2021, it is not used in any production explosives applications, though it is used as a precursor chemical in one method of production of TATB, another explosive.
HNB was experimentally used as a gas source for explosively pumped gas dynamic laser.[3] In this application, HNB and tetranitromethane are preferred to more conventional explosives because the explosion products CO2 and N2 are a simple enough mixture to simulate gas dynamic processes and quite similar to conventional gas dynamic laser medium. The water and hydrogen products of many other explosives could interfere with vibrational states of CO2 in this type of laser.
Preparation
During World War II, a method of synthesis of hexanitrobenzene was suggested in Germany, and the product was supposed to be manufactured on a semi-industrial scale according to the following scheme:
- C6H3(NO2)3 → C6H3(NHOH)3 (partial reduction)
- C6H3(NHOH)3 → C6(NO2)3(NHOH)3 (nitration)
- C6(NO2)3(NHOH)3 → C6(NO2)6 (oxidation)
Complete nitration of benzene is practically impossible because the nitro groups are deactivating groups for further nitration.
Additional properties
- Chapman-Jouget detonation pressure: 43 GPa
- Crystal Density: 2.01
See also
- ONC
- 4,4’-Dinitro-3,3’-diazenofuroxan (DDF)
- Octaazacubane (N8)
- Hexanitrohexaazaisowurtzitane (HNIW)
- Tetryl
- TNT
- RE factor
Notes
- ↑ Accurate determination of pair potentials for a CwHxNyOz system of molecules: A semiempirical method, Thiel et al., 1995
- 1 2 Bart, J. C. J. (1968). "The crystal structure of a modification of hexaphenylbenzene". Acta Crystallographica Section B. 24 (10): 1277–1287. doi:10.1107/S0567740868004176.
- ↑ Condensed explosive gas dynamic laser, United States Patent 4099142
References
- Heats of Formation and Chemical Compositions
- The synthesis and characterisation of halogen and nitro phenyl azide derivatives as highly energetic materials., PhD Thesis, Adam, D; 2001
- R. L. Atkins; R. A. Hollins; W. S. Wilson (1986). "Synthesis of polynitro compounds. Hexasubstituted benzenes". J. Org. Chem. 51 (17): 3261–3266. doi:10.1021/jo00367a003.
- A. T. Nielsen; R. L. Atkins; W. P. Norris (1979). "Oxidation of poly(nitro)anilines to poly(nitro)benzenes. Synthesis of hexanitrobenzene and pentanitrobenzene". J. Org. Chem. 44 (7): 1181–1182. doi:10.1021/jo01321a041.
- Z. A. Akopyan; Yu. T. Struchkov; V. G. Dashevskii (1966). "Crystal and molecular structure of hexanitrobenzene". Journal of Structural Chemistry. 7 (3): 385–392. doi:10.1007/BF00744430. S2CID 96053767.