 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /tæmˈsuːləsɪn/[1] tam-SOO-lə-sin |
| Trade names | Flomax, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698012 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (by mouth) |
| Metabolism | Liver |
| Elimination half-life | 9–13 hours |
| Excretion | 76% Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.109.780 |
| Chemical and physical data | |
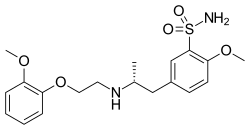
| Formula | C20H28N2O5S |
| Molar mass | 408.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |

Tamsulosin, sold under the brand name Flomax among others, is a medication used to treat symptomatic benign prostatic hyperplasia (BPH) and chronic prostatitis and to help with the passage of kidney stones.[5][6][7] The evidence for benefit with a kidney stone is better when the stone is larger.[7] It is taken by mouth.[5]
Common side effects include dizziness, headache, sleeplessness, nausea, blurry vision, and sexual problems.[8][5] Other side effects may include feeling lightheaded with standing due to changes in blood pressure, and angioedema.[8] Tamsulosin is an alpha blocker and works by relaxing muscles in the prostate.[9] Specifically it is an α1 adrenergic receptor blocker.[5]
Tamsulosin was approved for medical use in the United States in 1997.[5] It is available as a generic medication.[8] In 2021, it was the 24th most commonly prescribed medication in the United States, with more than 23 million prescriptions.[10][11]
Medical uses

Tamsulosin is primarily used for benign prostatic hyperplasia (BPH) and to help with the passage of kidney stones.[12][13] Tamsulosin, however, appears to be effective only for stones over 4 mm and less than 10 mm in size.[7]
Tamsulosin is also used as an add-on treatment for acute urinary retention. People may void more successfully after catheter removal if they are taking tamsulosin. People taking tamsulosin also are less likely to need recatheterization.[14]
Tamsulosin does not decrease the overall size of the prostate in men with BPH, and is not recommended for prevention of prostate cancer.[15]
Combination therapy
The results of the CombAT (combination of dutasteride (Avodart) and tamsulosin, under the brand name Duodart) trial in 2008 demonstrated that treatment with the combination of dutasteride and tamsulosin provides greater symptom benefits compared to monotherapy with either agent alone for treatment of benign prostatic hyperplasia.[16] The CombAT trial became the medication Jalyn. It was approved by the FDA on 14 June 2010.[17] This combination can be useful because it may take up to six months for symptomatic relief to be found when using 5-alpha-reductase inhibitors such as dutasteride compared to alpha-1 receptor blockers, which can provide relief in some cases within 48 hours.[18]
Adverse effects
- Eyes: People taking tamsulosin are prone to a complication known as floppy iris syndrome during cataract surgery. Adverse outcomes of the surgery are greatly reduced by the surgeon's prior knowledge of the person's history with this drug, and thus having the option of alternative techniques.[19]
- Severe hypotension.[20][21]
- Persons with cardiac conditions including hypotension, mechanical heart failure (valvular, pulmonary embolism, pericarditis), and congestive heart failure should be monitored carefully while taking tamsulosin.
- Alpha blockers, including prazosin, terazosin, doxazosin, or tamsulosin, do not appear to affect all-cause mortality in heart failure rehospitalization in those also receiving β-blockers.[22]
- Tamsulosin can also cause retrograde ejaculation, which occurs when semen is redirected to the urinary bladder instead of being ejaculated normally. This is because tamsulosin relaxes the muscles of the urethral sphincters, which are normally closed during ejaculation.[23]
Mechanism
Tamsulosin is a selective α1 receptor antagonist that has preferential selectivity for the α1A receptor in the prostate versus the α1B receptor in the blood vessels.[24]
When alpha 1 receptors in the bladder neck, prostate, ureter, and urethra are blocked, a relaxation in smooth muscle tissue results.[15] This mechanism decreases resistance to urinary flow, reduces discomfort associated with BPH, and facilitates passage of kidney stones.[15]
Brand names
Tamsulosin was first marketed in 1996 under the trade name Flomax. The U.S. patent expired in October 2009.[25] The U.S. Food and Drug Administration (FDA) approved generics in March 2010.[26] In 2010, tamsulosin was available as OTC medication in UK.[27]
It is marketed by various companies under licence, including Boehringer Ingelheim and CSL. Tamsulosin hydrochloride extended-release capsules are marketed under the trade names Urimax 0.4 (India),Tamlocept 0.4 (India), Flomax, Flomaxtra, Contiflo XL, bestflo, Mecir LP (France), Urimax, Pamsvax, and Pradif,[28] although generic, unmodified-release capsules are still approved and marketed in many countries (such as Canada). Generic extended-release tablets are marketed in most countries of the EEA.[29] In Mexico, it is marketed as Secotex and as Harnal D in Japan and Indonesia and as Harnal OCAS (oral controlled absorption system) in Thailand.[30] In Egypt,[31] Italy, Russia and Iceland, it is marketed under the trade name Omnic by Astellas Pharma Europe. The largest manufacturer of tamsulosin is Synthon BV (the Netherlands). Tamsulosin hydrochloride is marketed in Bangladesh under the trade names Uromax, Prostanil MR, Tamisol MR, and Tamsin.
References
- ↑ "Tamsulosin". Merriam-Webster.com Dictionary.
- ↑ "Flomaxtra XL, 400 micrograms, film-coated prolonged release tablet - Summary of Product Characteristics (SmPC)". (emc). 12 November 2019. Retrieved 2 December 2021.
- ↑ "Faramsil 400 microgram Prolonged-release Tablets - Summary of Product Characteristics (SmPC)". (emc). 4 November 2020. Retrieved 2 December 2021.
- ↑ "Flomax- tamsulosin hydrochloride capsule". DailyMed. Retrieved 2 December 2021.
- 1 2 3 4 5 "Tamsulosin Hydrochloride Monograph for Professionals". Drugs.com. AHFS. Retrieved 24 December 2018.
- ↑ "Prostatitis". NHS. 19 October 2017. Retrieved 24 December 2018.
- 1 2 3 Wang RC, Smith-Bindman R, Whitaker E, Neilson J, Allen IE, Stoller ML, Fahimi J (March 2017). "Effect of Tamsulosin on Stone Passage for Ureteral Stones: A Systematic Review and Meta-analysis". Annals of Emergency Medicine. 69 (3): 353–361.e3. doi:10.1016/j.annemergmed.2016.06.044. PMID 27616037.
- 1 2 3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 767. ISBN 9780857113382.
- ↑ Hutchison LC, Sleeper RB (2010). Fundamentals of Geriatric Pharmacotherapy: An Evidence-Based Approach. ASHP. p. 209. ISBN 9781585283057.
- ↑ "The Top 300 of 2021". ClinCalc. Retrieved 14 January 2024.
- ↑ "Tamsulosin - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ↑ "Tamsulosin Aids Stone Expulsion". Renal and Urology News. 7 January 2011.
- ↑ "Study Shows Use of Tamsulosin or Nifedipine Helps Patients to Clear Ureteral Stone Fragments Faster and Reduces Rate of Recurrence".
- ↑ Lucas MG, Stephenson TP, Nargund V (February 2005). "Tamsulosin in the management of patients in acute urinary retention from benign prostatic hyperplasia". BJU International. 95 (3): 354–357. doi:10.1111/j.1464-410X.2005.05299.x. PMID 15679793. S2CID 30254915.
- 1 2 3 Lewis SM, Dirksen SR, Heitkemper MM, Bucher LH (5 December 2013). Medical-surgical nursing : assessment and management of clinical problems (Ninth ed.). St. Louis, Missouri. ISBN 978-0-323-10089-2. OCLC 228373703.
{{cite book}}: CS1 maint: location missing publisher (link) - ↑ Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Morrill B, Montorsi F (February 2008). "The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study". The Journal of Urology. 179 (2): 616–21, discussion 621. doi:10.1016/j.juro.2007.09.084. PMID 18082216.
- ↑ FDA approval letter at FDA.gov
- ↑ Australian Medicines Handbook
- ↑ Medscape, Good Cataract Surgery Outcomes Possible in Intraoperative Floppy Iris Syndrome Due to Tamsulosin.
- ↑ Bird ST, Delaney JA, Brophy JM, Etminan M, Skeldon SC, Hartzema AG (November 2013). "Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40-85 years in the United States: risk window analyses using between and within patient methodology". BMJ. 347: f6320. doi:10.1136/bmj.f6320. PMC 3817852. PMID 24192967.
- ↑ Ramirez J (November 2013). "Severe hypotension associated with α blocker tamsulosin". BMJ. 347: f6492. doi:10.1136/bmj.f6492. hdl:10906/78488. PMID 24192968. S2CID 24324483.
- ↑ Page RL, O'Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, et al. (August 2016). "Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association". Circulation. 134 (6): e32–e69. doi:10.1161/CIR.0000000000000426. PMID 27400984.
- ↑ "Tamsulosin Side Effects". Drugs.com. Retrieved 27 April 2011.
- ↑ Shen H (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 13. ISBN 978-1-59541-101-3.
- ↑ "Flomax – Big Patent Expirations of 2010". 10 February 2010. Archived from the original on 22 April 2012. Retrieved 14 January 2012.
- ↑ "FDA Approves First Generic Tamsulosin to Treat Enlarged Prostate Gland" (Press release). U.S. Food and Drug Administration (FDA). 2 March 2010.
- ↑ "OTC tamsulosin for benign prostatic hyperplasia". Drug and Therapeutics Bulletin. 48 (10): 113–116. October 2010. doi:10.1136/dtb.2010.10.0052. PMID 20926447. S2CID 32141889.
- ↑ Magnanelli S, Vetere AM. "Pradif 0,4 Mg Capsule Rigide A Rilascio Prolungato". Torrinomedica.it. Retrieved 15 November 2012.
- ↑ "Tamsulosina Mylan 0,4 mg cápsulas duras de liberación modificada EFG" (PDF). cima.aemps.es. Retrieved 29 October 2018.
- ↑ "Drugs.com Database".
- ↑ "Novartis hits Astellas with transplant drug generic". Reuters. 11 August 2009. Retrieved 11 August 2009.