| Glaser coupling | |
|---|---|
| Named after | Carl Andreas Glaser |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | glaser-coupling |
| RSC ontology ID | RXNO:0000098 |
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is ammonia. The solvent is water or an alcohol. The reaction was first reported by Carl Andreas Glaser in 1869.[1][2] He suggested the following process for his way to diphenylbutadiyne:
- CuCl + PhC2H + NH3 → PhC2Cu + NH4Cl
- 4 PhC2Cu + O2 → 2PhC2C2Ph + 2Cu2O
Modifications
Eglinton reaction
| Eglinton reaction | |
|---|---|
| Named after | Geoffrey Eglinton |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | eglinton-reaction |
| RSC ontology ID | RXNO:0000099 |
In the related Eglinton reaction two terminal alkynes are coupled by a copper(II) salt such as cupric acetate.[3]
The oxidative coupling of alkynes has been used to synthesize a number of fungal antibiotics. The stoichiometry is represented by this highly simplified scheme:[4]
Such reactions proceed via copper(I)-alkyne complexes.
This methodology was used in the synthesis of cyclooctadecanonaene.[5] Another example is the synthesis of diphenylbutadiyne from phenylacetylene.[6]
Hay coupling
The Hay coupling is variant of the Glaser coupling. It relies on the TMEDA complex of copper(I) chloride to activate the terminal alkyne. Oxygen (air) is used in the Hay variant to oxidize catalytic amounts of Cu(I) to Cu(II) throughout the reaction, as opposed to a stoichiometric amount of Cu(II) used in the Eglington variant.[7] The Hay coupling of trimethylsilylacetylene gives the butadiyne derivative.[8]
Scope
In 1882 Adolf von Baeyer used the method to prepare 1,4-bis(2-nitrophenyl)butadiyne, en route to indigo dye.[9][10]
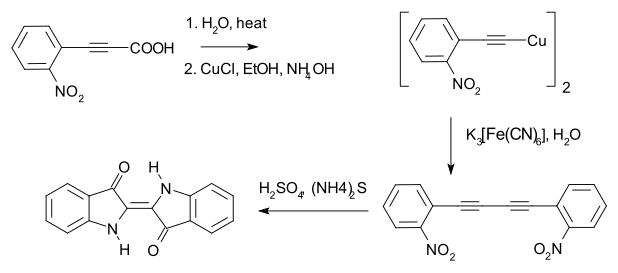
Shortly afterwards, Baeyer reported a different route to indigo, now known as the Baeyer–Drewson indigo synthesis.
See also
- Cadiot–Chodkiewicz coupling - Another alkyne coupling reaction catalysed by copper(I).
- Sonogashira coupling - Pd/Cu catalysed coupling of an alkyne and an aryl or vinyl halide
- Castro–Stephens coupling - A cross-coupling reaction between a copper(I) acetylide and an aryl halide
- Fritsch–Buttenberg–Wiechell rearrangement - can also form diynes
References
- ↑ Glaser, Carl (1870). "Untersuchungen über einige Derivate der Zimmtsäure" [Studies on some derivatives of cinnamic acid]. Annalen der Chemie und Pharmacie (in German). 154 (2): 137–171. doi:10.1002/jlac.18701540202.
- ↑ Glaser, C. (1869). "Beiträge zur Kenntniss des Acetenylbenzols". Berichte der Deutschen Chemischen Gesellschaft. 2 (1): 422–424. doi:10.1002/cber.186900201183.
- ↑ Eglinton, G.; Galbraith, A. R. (1959). "Macrocyclic Acetglenic Compounds. Part I. cyclo-Tetradeca-1:3-diyne and Related Compounds". J. Chem. Soc.: 889. doi:10.1039/JR9590000889.
- ↑ Eglinton, G.; McRae, W. Adv. Org. Chem. 1963, 4, 225.
- ↑ K. Stöckel and F. Sondheimer (1974). "[18]Annulene". Organic Syntheses. 54: 1. doi:10.15227/orgsyn.054.0001.
- ↑ I. D. Campbell and G. Eglinton (1965). "Diphenyldiacetylene". Organic Syntheses. 45: 39. doi:10.15227/orgsyn.045.0039.
- ↑ Hay, Allan S. (1962). "Oxidative Coupling of Acetylenes. II". The Journal of Organic Chemistry. 27 (9): 3320–3321. doi:10.1021/jo01056a511.
- ↑ Graham E. Jones, David A. Kendrick, Andrew B. Holmes (1987). "1,4-Bis(trimethylsilyl)buta-1,3-diyne". Organic Syntheses. 65: 52. doi:10.15227/orgsyn.065.0052.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Baeyer, Adolf (1882). "Ueber die Verbindungen der Indigogruppe". Berichte der Deutschen Chemischen Gesellschaft. 15 (1): 50–56. doi:10.1002/cber.18820150116.
- ↑ Johansson Seechurn, Carin C. C.; Kitching, Matthew O.; Colacot, Thomas J.; Snieckus, Victor (21 May 2012). "Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize". Angewandte Chemie International Edition. 51 (21): 5062–5085. doi:10.1002/anie.201107017. PMID 22573393.
