 | |
 | |
| Names | |
|---|---|
| IUPAC name
meso-Erythritol | |
| Systematic IUPAC name
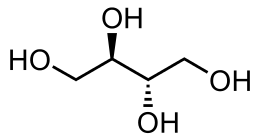
(2R,3S)-Butane-1,2,3,4-tetrol | |
| Other names
(2R,3S)-Butane-1,2,3,4-tetraol (not recommended) | |
| Identifiers | |
3D model (JSmol) |
|
| 1719753 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.217 |
| E number | E968 (glazing agents, ...) |
| 82499 | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H10O4 | |
| Molar mass | 122.120 g·mol−1 |
| Density | 1.45 g/cm3 |
| Melting point | 121 °C (250 °F; 394 K) |
| Boiling point | 329 to 331 °C (624 to 628 °F; 602 to 604 K) |
| 61% w/w (25 °C)[1] | |
| −73.80·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
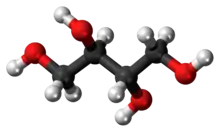
Erythritol (/ɪˈrɪθrɪtɒl/, US: /-tɔːl, -toʊl/)[2] is an organic compound, the naturally occurring achiral meso four-carbon sugar alcohol (or polyol).[3] It is the reduced form of either D- or L-erythrose and one of the two reduced forms of erythrulose. It is used as a food additive and sugar substitute. It is synthesized from corn using enzymes and fermentation. Its formula is C
4H
10O
4, or HO(CH2)(CHOH)2(CH2)OH.
Erythritol is 60–70% as sweet as sucrose (table sugar). However, erythritol is almost completely noncaloric,[4] and does not affect blood sugar[5] or cause tooth decay.[6] Japanese companies pioneered the commercial development of erythritol as a sweetener in the 1990s.
Etymology
The name "erythritol" derives from the Greek word for the color red (erythros or ἐρυθρός). This is the case even though erythritol is almost always found in the form of white crystals or powder and it does not turn red as a result of chemical reactions. The name "erythritol" comes from erythrin, a closely related compound, which turns red upon oxidation.[7]
History
Erythritol was discovered in 1848 by Scottish chemist John Stenhouse[8] and first isolated in 1852. In 1950 it was found in blackstrap molasses that was fermented by yeast, and it became commercialized as a sugar alcohol in the 1990s in Japan.[9]

Occurrence
Erythritol occurs naturally in some fruit and fermented foods.[10] It also occurs in human body fluids, such as eye lens tissue, serum, plasma, fetal fluid, and urine.[11]
Uses

Since 1990, erythritol has had a history of safe use as a sweetener and flavor-enhancer in food and beverage products, and is approved for use by government regulatory agencies of more than 60 countries.[12] Beverage categories for its use are coffee and tea, liquid dietary supplements, juice blends, soft drinks, and flavored water product variations, with foods including confections, biscuits and cookies, tabletop sweeteners, and sugar-free chewing gum.[12] The mild sweetness of erythritol allows for a volume-for-volume replacement of sugar, whereas sweeter sugar substitutes need fillers that result in a noticeably different texture in baked products.[13]
Absorption and excretion
Erythritol is absorbed rapidly into the blood, with peak amounts occurring in under two hours; the majority of an oral dose (80 to 90%) is excreted unchanged in the urine within 24 hours.[12]
Safety
In 2023, European Food Safety Authority reassessed the safety of erythritol and lowered the recommended daily intake limit to 0.5 grams per kg body weight,[14] which equates to 35 g for an average adult (70 kg). This lower limit was set to "safeguard against its laxative effect and to mitigate against long-term effects, such as electrolyte imbalance arising from prolonged exposure to erythritol-induced diarrhea."[14]
Previously, in 2015, scientists assessed doses for erythritol where symptoms of mild gastrointestinal upset occurred, such as nausea, excess flatus, abdominal bloating or pain, and stool frequency. At a content of 1.6% in beverages it was not considered to have a laxative effect.[12] The upper limit of tolerance was 0.78 and 0.71 grams per kg body weight in adults and children, respectively.[12]
Dietary and metabolic aspects
Caloric value and labeling
Nutritional labeling of erythritol in food products varies from country to country. Some countries, such as Japan and the European Union (EU), label it as zero-calorie.[15]
Under U.S. Food and Drug Administration (FDA) labeling requirements, it has a caloric value of 0.2 calories per gram (95% less than sugar and other carbohydrates). The FDA has not made its own determination regarding the generally recognized as safe (GRAS) status of erythritol, but has accepted the conclusion that erythritol is GRAS as submitted to it by several food manufacturers.[16]
Human digestion
In the body, most erythritol is absorbed into the bloodstream in the small intestine, and then for the most part excreted unchanged in the urine. About 10% enters the colon.[17]
In small doses, erythritol does not normally cause laxative effects and gas or bloating, as are often experienced after consumption of other sugar alcohols (such as maltitol, sorbitol, xylitol, and lactitol).[18] About 90% is absorbed before it enters the large intestine, and since erythritol is not digested by intestinal bacteria, the remaining 10% is excreted in the feces.[17]
Large doses can cause nausea, stomach rumbling and watery feces.[19] In males, doses greater than 0.66 g/kg body weight, and in females, doses greater than 0.8 g/kg body weight, will cause laxation,[20] and diarrhea in higher doses (over 50 grams (1.8 oz)).[19] Rarely, erythritol can cause allergic hives (urticaria).[21]
Blood sugar and insulin levels
Erythritol has no effect on blood sugar or blood insulin levels[22][23] and therefore may become an effective substitute for sugar for diabetics.[9] The glycemic index (GI) of erythritol is 0% of the GI for glucose and the insulin index (II) is 2% of the II for glucose.[24]
Oral bacteria
Erythritol is tooth-friendly; it cannot be metabolized by oral bacteria, so it does not contribute to tooth decay.[6][23] In addition, erythritol, similarly to xylitol, has antibacterial effects against streptococci bacteria, reduces dental plaque, and may be protective against tooth decay.[23]
Manufacturing
Erythritol is manufactured using enzymatic hydrolysis of the starch from corn to generate glucose.[25] Glucose is then fermented with yeast or another fungus to produce erythritol. A genetically engineered mutant form of Yarrowia lipolytica, a yeast, has been optimized for erythritol production by fermentation, using glycerol as a carbon source and high osmotic pressure to increase yields up to 62%.[11]
Chemical properties
Heat of solution
Erythritol has a strong cooling effect (endothermic, or positive heat of solution)[26] when it dissolves in water, which is often compared with the cooling effect of mint flavors. The cooling effect is present only when erythritol is not already dissolved in water, a situation that might be experienced in an erythritol-sweetened frosting, chocolate bar, chewing gum, or hard candy. The cooling effect of erythritol is very similar to that of xylitol and among the strongest cooling effects of all sugar alcohols.[27] Erythritol has a pKa of 13.903 at 18 °C.[28]
Biological properties
According to a 2014 study,[29] erythritol functions as an insecticide toxic to the fruit fly Drosophila melanogaster, impairing motor ability and reducing longevity even when nutritive sugars were available.
Erythritol is preferentially used by the Brucella spp. The presence of erythritol in the placentas of goats, cattle, and pigs has been proposed as an explanation for the accumulation of Brucella bacteria found at these sites.[30]
Synonyms
In the 19th and early 20th centuries, several synonyms were in use for erythritol: erythrol, erythrite, erythoglucin, eryglucin, erythromannite and phycite.[31] Zerose is a tradename for erythritol.[32]
See also
- Erythritol tetranitrate
- Pentaerythritol
- Threitol, the diastereomer of erythritol
References
- ↑ O'Neil M, ed. (2006). The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (14th ed.). Merck. p. 629. ISBN 978-0-911910-00-1.
- ↑ "erythritol". CollinsDictionary.com. HarperCollins. Retrieved 2023-06-29.
- ↑ Rzechonek DA, Dobrowolski A, Rymowicz W, Mirończuk AM (June 2018). "Recent advances in biological production of erythritol". Critical Reviews in Biotechnology. 38 (4): 620–633. doi:10.1080/07388551.2017.1380598. PMID 28954540. S2CID 3075870.
- ↑ Vasudevan DM (2013). Textbook of biochemistry for medical students. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd. p. 81. ISBN 978-93-5090-530-2.
- ↑ Moon HJ, Jeya M, Kim IW, Lee JK (April 2010). "Biotechnological production of erythritol and its applications". Applied Microbiology and Biotechnology. 86 (4): 1017–1025. doi:10.1007/s00253-010-2496-4. PMID 20186409. S2CID 9560435.
- 1 2 Kawanabe J, Hirasawa M, Takeuchi T, Oda T, Ikeda T (1992). "Noncariogenicity of erythritol as a substrate". Caries Research. 26 (5): 358–362. doi:10.1159/000261468. PMID 1468100.
- ↑ Senning, Alexander (2019). The Etymology of Chemical Names in Chemical Nomenclature: Tradition and Convenience vs. Rationality. De Gruyter. p. 85. ISBN 9783110611069. Retrieved 9 March 2023.
- ↑ The discovery of erythritol, which Stenhouse called "erythroglucin", was announced in: Stenhouse J (January 1, 1848). "Examination of the proximate principles of some of the lichens". Philosophical Transactions of the Royal Society of London. 138: 63–89, see especially p. 76. doi:10.1098/rstl.1848.0004. S2CID 83653513.
- 1 2 Boesten DM, den Hartog GJ, de Cock P (2015). "Health effects of erythritol". Nutrafoods. 14 (3): 3–9. doi:10.1007/s13749-014-0067-5.
- ↑ Shindou T, Sasaki, Y, Miki H, Eguchi T, Hagiwara K, Ichikawa T (1988). "Determination of erythritol in fermented foods by high performance liquid chromatography" (pdf). Shokuhin Eiseigaku Zasshi. 29 (6): 419–22. doi:10.3358/shokueishi.29.419.
- 1 2 Carly F, Fickers P (July 2018). "Erythritol production by yeasts: a snapshot of current knowledge". Yeast. 35 (7): 455–463. doi:10.1002/yea.3306. PMID 29322598.
- 1 2 3 4 5 Scientific Panel on Food Additives and Nutrient Sources Added to Food, European Food Safety Authority (2015). "Scientific Opinion on the safety of the proposed extension of use of erythritol (E 968) as a food additive". EFSA Journal. 13 (3): 4033. doi:10.2903/j.efsa.2015.4033. ISSN 1831-4732., Quote: "In 2003, the European Union (EU) Scientific Committee on Food (SCF) concluded that erythritol is safe for use in foods. [...] the SCF opinion stated that the laxative threshold may be exceeded, especially by young consumers, [...] the ANS Panel concluded that the acute bolus consumption of erythritol via non-alcoholic beverages at a maximum level of 1.6 % would not raise concerns for laxation."
- ↑ Regnat K, Mach RL, Mach-Aigner AR (January 2018). "Erythritol as sweetener-wherefrom and whereto?". Applied Microbiology and Biotechnology. 102 (2): 587–595. doi:10.1007/s00253-017-8654-1. PMC 5756564. PMID 29196787.
- 1 2 "Europe: Erythritol Laxative Effect, Lead Levels Concerning". Medscape. 2023-11-22. Retrieved 2024-01-01.
- ↑ (2008) European Commission Directive 2008/100/EC. Quote: "Erythritol is a polyol, and according to the current rules as provided for in Article 5(1) of Directive 90/496/EEC, its energy would be calculated using the conversion factor for polyols, namely 10 kJ/g (2,4 kcal/g). Using this energy conversion factor would not fully inform the consumer about the reduced energy value of a product achieved by the use of erythritol in its manufacture. The Scientific Committee on Food in its opinion on erythritol, expressed on March 5, 2003, noted that the energy provided by erythritol was less than 0,9 kJ/g (less than 0,2 kcal/g). Therefore it is appropriate to adopt a suitable energy conversion factor for erythritol. Current regulations (Reg. (EC) 1169/2011) preserve this conversion factor at 0 kcal/g for energy value calculation purposes."
- ↑ "GRAS notices: erythritol". US Food and Drug Administration. 16 November 2018. Retrieved 8 December 2018.
- 1 2 Arrigoni E, Brouns F, Amadò R (November 2005). "Human gut microbiota does not ferment erythritol". The British Journal of Nutrition. 94 (5): 643–646. doi:10.1079/BJN20051546. hdl:20.500.11850/31086. PMID 16277764.
- ↑ Munro IC, Berndt WO, Borzelleca JF, Flamm G, Lynch BS, Kennepohl E, et al. (December 1998). "Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data". Food and Chemical Toxicology. 36 (12): 1139–1174. doi:10.1016/S0278-6915(98)00091-X. PMID 9862657.
- 1 2 Storey D, Lee A, Bornet F, Brouns F (March 2007). "Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid". European Journal of Clinical Nutrition. 61 (3): 349–354. doi:10.1038/sj.ejcn.1602532. PMID 16988647. S2CID 10228622.
- ↑ Mäkinen KK (2016). "Gastrointestinal Disturbances Associated with the Consumption of Sugar Alcohols with Special Consideration of Xylitol: Scientific Review and Instructions for Dentists and Other Health-Care Professionals". International Journal of Dentistry. 2016: 5967907. doi:10.1155/2016/5967907. PMC 5093271. PMID 27840639.
- ↑ Hino H, Kasai S, Hattori N, Kenjo K (March 2000). "A case of allergic urticaria caused by erythritol". The Journal of Dermatology. 27 (3): 163–165. doi:10.1111/j.1346-8138.2000.tb02143.x. PMID 10774141. S2CID 40328472.
- ↑ Munro IC, Berndt WO, Borzelleca JF, Flamm G, Lynch BS, Kennepohl E, et al. (December 1998). "Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data". Food and Chemical Toxicology. 36 (12): 1139–1174. doi:10.1016/S0278-6915(98)00091-X. PMID 9862657.
- 1 2 3 de Cock P (2012). "Erythritol". Sweeteners and Sugar Alternatives in Food Technology. pp. 213–41. doi:10.1002/9781118373941.ch10. ISBN 9781118373941.
- ↑ Livesey G (December 2003). "Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties". Nutrition Research Reviews. 16 (2): 163–191. doi:10.1079/NRR200371. PMID 19087388. S2CID 4541994.
- ↑ Piccirillo C (January 28, 2014). "How Is Erythritol Made? Manufacture of a Low-Calorie Sugar Substitute". Decoded Science. Archived from the original on October 30, 2016. Retrieved July 18, 2016.
- ↑ Wohlfarth C (2006). CRC handbook of enthalpy data of polymer-solvent systems. CRC / Taylor & Francis. p. 3. ISBN 978-0-8493-9361-7.
- ↑ Jasra RV, Ahluwalia JC (1982). "Enthalpies of Solution, Partial Molal Heat Capacities and Apparent Molal Volumes of Sugars and Polyols in Water". Journal of Solution Chemistry. 11 (5): 325–38. doi:10.1007/BF00649291. ISSN 1572-8927. S2CID 93845620.
- ↑ O'Neil MJ (2006). "Erythritol". The Merck Index – an Encyclopedia of Chemicals, Drugs, and Biologicals: 629.
- ↑ Baudier KM, Kaschock-Marenda SD, Patel N, Diangelus KL, O'Donnell S, Marenda DR (2014). "Erythritol, a non-nutritive sugar alcohol sweetener and the main component of truvia®, is a palatable ingested insecticide". PLOS ONE. 9 (6): e98949. Bibcode:2014PLoSO...998949B. doi:10.1371/journal.pone.0098949. PMC 4045977. PMID 24896294.
- ↑ Petersen E, Rajashekara G, Sanakkayala N, Eskra L, Harms J, Splitter G (June 2013). "Erythritol triggers expression of virulence traits in Brucella melitensis". Microbes and Infection. 15 (6–7): 440–449. doi:10.1016/j.micinf.2013.02.002. PMC 3686989. PMID 23421980.
- ↑ Hart E (1892). "A list of words whose use should be avoided in favor of the accompanying synonyms". Journal of Analytical and Applied Chemistry. 6: 160.
- ↑ "Cargill unveils new products featuring Zerose natural sweetener". New Hope Network. 9 March 2010. Retrieved 13 November 2018.
