 | |
| Names | |
|---|---|
| Preferred IUPAC name
Disodium 2,2′-[(E)-ethene-1,2-diyl]bis(5-nitrobenzene-1-sulfonate) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.956 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H8N2Na2O10S2 | |
| Molar mass | 474.335 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
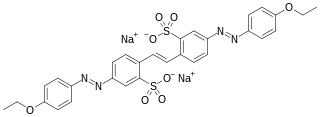
Disodium 4,4′-dinitrostilbene-2,2′-disulfonate is an organic compound with the formula (O2NC6H3(SO3Na)CH)2. This salt is a common precursor to a variety of textile dyes and optical brighteners
Preparation and reactions
The synthesis of disodium 4,4′-dinitrostilbene-2,2′-disulfonate begins with sulfonation of 4-nitrotoluene. This reaction affords 4-nitrotoluene-2-sulfonic acid. Oxidation of this species with sodium hypochlorite yields the disodium salt of 4,4′-dinitrostilbene-2,2′-disulfonic acid.[1] The product is useful as its reaction with aniline derivatives results in the formation of azo dyes. Commercially important dyes derived from this compound include Direct Red 76, Direct Brown 78, and Direct Orange 40.[2] Reduction gives 4,4′-diamino-2,2′-stilbenedisulfonic acid, which is a common optical brightener.
History
Arthur Green and André Wahl first reported the formation of disodium 4,4'-dinitrostilbene-2,2'-disulfonate using sodium hypochlorite.[3][4]
References
- ↑ Cumming, William M.; Hopper, I. Vance; Wheeler, T. Sherlock (1926). "Preparation 294.—Dinitro-Stilbene-Disulphonic Acid (Na salt)". Systematic Organic Chemistry: Modern Methods of Preparation and Estimation. New York: D. Van Nostrand Company. p. 314.
- ↑ Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys (2005). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 3527306730.
- ↑ Green, Arthur G.; Wahl, André R. (1897). "Ueber die Oxydation von Paranitrotoluolsulfosäure" [On the oxidation of para-nitrotoluenesulfonic acid]. Ber. Dtsch. Chem. Ges. (in German). 30 (3): 3097–3101. doi:10.1002/cber.189703003128.
- ↑ Green, Arthur G.; Wahl, André R. (1898). "Ueber die Oxydation der Paranitrotoluolsulfosäure" [On the oxidation of para-nitrotoluenesulfonic acid]. Ber. Dtsch. Chem. Ges. (in German). 31 (1): 1078–1080. doi:10.1002/cber.189803101195.