 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
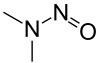
| Preferred IUPAC name
N,N-Dimethylnitrous amide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.500 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Dimethylnitrosamine | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3382 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H6N2O | |||
| Molar mass | 74.083 g·mol−1 | ||
| Appearance | Yellow oil[1] | ||
| Odor | faint, characteristic[1] | ||
| Density | 1.005 g/mL | ||
| Boiling point | 153.1 °C; 307.5 °F; 426.2 K | ||
| 290 mg/ml (at 20 °C) | |||
| log P | −0.496 | ||
| Vapor pressure | 700 Pa (at 20 °C) | ||
Refractive index (nD) |
1.437 | ||
| Thermochemistry | |||
Std enthalpy of combustion (ΔcH⦵298) |
1.65 MJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Known carcinogen,[1] extremely toxic | ||
| GHS labelling: | |||
   | |||
| Danger | |||
| H301, H330, H350, H372, H411 | |||
| P260, P273, P284, P301+P310, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 61.0 °C (141.8 °F; 334.1 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
37.0 mg/kg (oral, rat) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
OSHA-Regulated Carcinogen[1] | ||
REL (Recommended) |
Ca[1] | ||
IDLH (Immediate danger) |
Ca [N.D.][1] | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of N-nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in laboratory animals.[2]
Occurrence
Drinking water
Of more general concern, NDMA can be produced by water treatment by chlorination or chloramination. The question is the level at which it is produced. In the U.S. state of California, the allowable level is 10 nanograms/liter. The Canadian province of Ontario set the standard at 9 ng/L. The potential problem is greater for recycled water that can contain dimethylamine.[3] Further, NDMA can form or be leached during treatment of water by anion exchange resins.[4]
Contamination of drinking water with NDMA is of particular concern due to the minute concentrations at which it is harmful, the difficulty in detecting it at these concentrations, and to the difficulty in removing it from drinking water. It does not readily biodegrade, adsorb, or volatilize. As such, it cannot be removed by activated carbon and travels easily through soils.
Relatively high levels of UV radiation in the 200 to 260 nm range breaks the N–N bond. Thus, it can be used to degrade NDMA. Additionally, reverse osmosis removes approximately 50% of NDMA.[5]
Cured meat
NDMA is found at low levels in numerous items of human consumption, including cured meat, fish, beer, as well as during use of tobacco products and the inhalation of tobacco smoke.[4][6]
Rocket fuel
Unsymmetrical dimethylhydrazine, a rocket fuel, is a highly effective precursor to NDMA:
- (CH3)2NNH2 + 2 O → (CH3)2NNO + H2O
Groundwater near rocket launch sites often has high levels of NDMA.[5]
Regulation
United States
The United States Environmental Protection Agency (EPA) determined that the maximal admissible concentration of NDMA in drinking water is 7 ng/L.[7] As of July 2020, the EPA has not set a regulatory maximal contaminant level (MCL) for drinking water. At high doses, it is a "potent hepatotoxin that can cause fibrosis of the liver" in rats.[8] The induction of liver tumors in rats after chronic exposure to low doses is well documented.[9] Its toxic effects on humans are inferred from animal experiments, but not well-established experimentally.
It is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities that produce, store, or use it in significant quantities.[10]
European Union
In July 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued an opinion requiring companies to take measures to limit the presence of nitrosamines in human medicines as far as possible and to ensure levels of these impurities do not exceed set limits.[11][12] Nitrosamines are classified as probable human carcinogens (substances that could cause cancer). The limits for nitrosamines in medicines have been set using internationally agreed standards (ICH M7(R1)) based on lifetime exposure.[11] Generally, people should not be exposed to a lifetime risk of cancer exceeding 1 in 100,000 from nitrosamines in their medicines.[11] EU regulators first became aware of nitrosamines in medicines in mid-2018, and took regulatory actions, including recalling medicines and stopping the use of active substances from certain manufacturers.[11] A subsequent CHMP review of sartan blood pressure medicines in 2019, led to new requirements for the manufacture of sartans, while its 2020 review of ranitidine recommended an EU-wide suspension of ranitidine medicines.[11]
Chemistry
The C2N2O core of NDMA is planar, as established by X-ray crystallography. The central nitrogen is bound to two methyl groups and the NO group with bond angles of 120°. The N-N and N-O distances are 1.32 and 1.26 Å, respectively.[13]
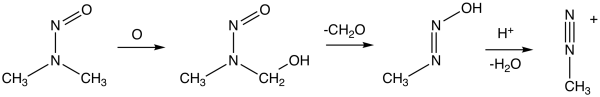
NDMA forms from a variety of dimethylamine-containing compounds, e.g. hydrolysis of dimethylformamide. Dimethylamine is susceptible to oxidation to unsymmetrical dimethylhydrazine, which air-oxidizes to NDMA.[14]
In the laboratory, NDMA can be synthesised by the reaction of nitrous acid with dimethylamine:
- HONO + (CH3)2NH → (CH3)2NNO + H2O
The mechanism of its carcinogenicity involves metabolic activation steps resulting in the formation of methyl diazonium, an alkylating agent.[2]
As a poison
Several incidents in which NDMA was used intentionally to poison another person have garnered media attention. In 1978, a teacher in Ulm, Germany, was sentenced to life in prison for trying to murder his wife by poisoning jam with NDMA and feeding it to her. Both the wife and the teacher later died from liver failure.[15][16]
In 1978, Steven Roy Harper spiked lemonade with NDMA at the Johnson family home in Omaha, Nebraska. The incident resulted in the deaths of 30-year-old Duane Johnson and 11-month-old Chad Shelton. For his crime, Harper was sentenced to death, but committed suicide in prison before his execution could be carried out.[17]
In the 2013 Fudan poisoning case, Huang Yang, a postgraduate medical student at Fudan University, was the victim of a poisoning in Shanghai, China. Huang was poisoned by his roommate Lin Senhao, who had placed NDMA into the water cooler in their dormitory. Lin claimed that he only did this as an April Fool's joke. He received a death sentence, and was executed in 2015.[18]
In 2018, NDMA was used in an attempted poisoning at Queen's University in Kingston, Canada.[19]
Drug contamination
In 2018, and then again in 2019, various brands of valsartan were recalled because of contamination with NDMA.[20][21] In 2019, ranitidine was recalled across the world due to contamination with NDMA.[22] In December 2019, the FDA began testing samples of the diabetes drug metformin for NDMA.[23] The FDA announcement followed a recall of three versions of metformin in Singapore, and the European Medicines Agency's request that manufacturers test for NDMA.[24][25]
In September 2019, N-nitrosodimethylamine was discovered in ranitidine products from a number of manufacturers, resulting in recalls.[26][27][28][29][30][31] In April 2020, ranitidine was withdrawn from the United States market, suspended in the European Union, and suspended in Australia due to concerns about NDMA.[28][32][33][34][35]
In August 2021, a class 2 medicines recall was issued for a batch of metformin hydrochloride 500 mg/5ml Oral Solution from Rosemont Pharmaceuticals Limited, which was first distributed in December 2020, due to the identification of higher than acceptable levels of NDMA.[36]
Effect on biological systems
A study has shown that NDMA perturbs arginine biosynthesis, mitochondrial genome maintenance and DNA damage repair in yeast.[37]
References
- 1 2 3 4 5 6 NIOSH Pocket Guide to Chemical Hazards. "#0461". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 Tricker, A.R.; Preussmann, R. (1991). "Carcinogenic N-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential". Mutation Research/Genetic Toxicology. 259 (3–4): 277–289. doi:10.1016/0165-1218(91)90123-4. PMID 2017213.
- ↑ David L. Sedlak; Rula A. Deeb; Elisabeth L. Hawley; William A. Mitch; Timothy D. Durbin; Sam Mowbray; Steve Carr (2005). "Sources and Fate of Nitrosodimethylamine and Its Precursors in Municipal Wastewater Treatment Plants". Water Environment Research. 77 (1, Emerging Micropollutants in Treatment Systems (Jan.–Feb. 2005)): 32–39. doi:10.2175/106143005X41591. JSTOR 25045835. PMID 15765933. S2CID 9690388.
- 1 2 Najm, I.; Trussell, R. R. (2001). "NDMA Formation in Water and Wastewater". Journal of the American Water Works Association. 93 (2): 92–99. doi:10.1002/j.1551-8833.2001.tb09129.x. ISSN 0003-150X. S2CID 93202942.
- 1 2 Mitch, W. A.; Sharp, J. O.; Trussell, R. R.; Valentine, R. L.; Alvarez-Cohen, L.; Sedlak, D. L. (2003). "N-Nitrosodimethylamine (NDMA) as a Drinking Water Contaminant: A Review". Environmental Engineering Science. 20 (5): 389–404. CiteSeerX 10.1.1.184.204. doi:10.1089/109287503768335896.
- ↑ Hecht, Stephen S. (1998). "Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines†". Chemical Research in Toxicology. 11 (6): 559–603. doi:10.1021/tx980005y. PMID 9625726.
- ↑ Andrzejewski, P.; Kasprzyk-Hordern, B.; Nawrocki, J. (2005). "The hazard of N-nitrosodimethylamine (NDMA) formation during water disinfection with strong oxidants". Desalination. 176 (1–3): 37–45. doi:10.1016/j.desal.2004.11.009.
- ↑ George, J.; Rao, K. R.; Stern, R.; Chandrakasan, G. (2001). "Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen". Toxicology. 156 (2–3): 129–138. doi:10.1016/S0300-483X(00)00352-8. PMID 11164615.
- ↑ Peto, R.; Gray, R.; Brantom, P.; Grasso, P. (1991). "Dose and Time Relationships for Tumor Induction in the Liver and Esophagus of 4080 Inbred Rats by Chronic Ingestion of N-Nitrosodiethylamine or N-Nitrosodimethylamine" (PDF). Cancer Research. 51 (23 Part 2): 6452–6469. PMID 1933907.
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
- 1 2 3 4 5 "EMA finalises opinion on presence of nitrosamines in medicines". European Medicines Agency (EMA) (Press release). 9 July 2020. Retrieved 11 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Nitrosamine impurities". European Medicines Agency (EMA). 23 October 2019. Retrieved 11 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Krebs, Bernt; Mandt, Jürgen (1975). "Kristallstruktur des N-Nitrosodimethylamins". Chemische Berichte. 108 (4): 1130–1137. doi:10.1002/cber.19751080419.
- ↑ Mitch, William A.; Sedlak, David L. (2002). "Formation of N-Nitrosodimethylamine (NDMA) from Dimethylamine during Chlorination". Environmental Science & Technology. 36 (4): 588–595. Bibcode:2002EnST...36..588M. doi:10.1021/es010684q. PMID 11878371.
- ↑ Ein teuflischer Plan: Tod aus dem Marmeladeglas (archived from the original, in German).
- ↑ Karsten Strey: "Die Welt der Gifte", Lehmanns, 2. Edition p. 193 (in German).
- ↑ Roueché, Berton (January 25, 1982). "The Prognosis for this Patient is Horrible". The New Yorker. pp. 57–71. Retrieved 2 July 2021.
- ↑ "15 days log in hospital". Archived from the original on 2014-01-09. Retrieved 2013-04-30.
- ↑ "Man admits poisoning fellow researcher in Kingston". The Kingston Whig Standard. 1 December 1969. Retrieved 2 July 2021.
- ↑ "FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan)". U.S. Food and Drug Administration (FDA). November 7, 2019. Retrieved November 15, 2019.
- ↑ "EMA reviewing medicines containing valsartan from Zhejiang Huahai following detection of an impurity: some being recalled across the EU". European Medicines Agency (EMA) (Press release). September 17, 2018. Retrieved December 3, 2019. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ BBC Staff (September 29, 2019). "Sale of heartburn drug suspended over cancer fears". BBC News. Retrieved December 3, 2019.
- ↑ "FDA Updates and Press Announcements on NDMA in Metformin". U.S. Food and Drug Administration (FDA). 2 July 2020. Retrieved 11 July 2020.
- ↑ Lauerman, John (December 4, 2019). "Diabetes Drugs Latest to Be Targeted for Carcinogen Scrutiny". Bloomberg Business. Retrieved January 5, 2020.
- ↑ Koenig, D. (December 6, 2019). "FDA Investigating Metformin for Possible Carcinogen". Medscape. Retrieved December 14, 2019.
- ↑ "Health Canada assessing NDMA in ranitidine". Health Canada. 13 September 2019. Archived from the original on 26 September 2019. Retrieved 26 September 2019.
- ↑ "Statement alerting patients and health care professionals of NDMA found in samples of ranitidine". U.S. Food and Drug Administration (FDA). 13 September 2019. Archived from the original on 26 September 2019. Retrieved 26 September 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "Questions and Answers: NDMA impurities in ranitidine (commonly known as Zantac)". U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 24 October 2019. Retrieved 23 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "EMA to provide guidance on avoiding nitrosamines in human medicines". European Medicines Agency (EMA) (Press release). 13 September 2019. Retrieved 19 September 2019. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "EMA to review ranitidine medicines following detection of NDMA". European Medicines Agency (EMA) (Press release). 13 September 2019. Retrieved 19 September 2019. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "FDA updates and press announcements on NDMA in Zantac (ranitidine)". U.S. Food and Drug Administration (FDA). 28 October 2019. Archived from the original on 29 October 2019. Retrieved 28 October 2019.
FDA observed the testing method used by a third-party laboratory uses higher temperatures. The higher temperatures generated very high levels of NDMA from ranitidine products because of the test procedure. FDA published the method for testing angiotensin II receptor blockers (ARBs) for nitrosamine impurities. That method is not suitable for testing ranitidine because heating the sample generates NDMA.
FDA recommends using an LC-HRMS testing protocol to test samples of ranitidine. FDA's LC-HRMS testing method does not use elevated temperatures and has shown the presence of much lower levels of NDMA in ranitidine medicines than reported by the third-party laboratory. International regulators using similar LC-MS testing methods have also shown the presence of low levels of NDMA in ranitidine samples. This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA Requests Removal of All Ranitidine Products (Zantac) from the Market". U.S. Food and Drug Administration (Press release). 1 April 2020. Retrieved 1 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Suspension of ranitidine medicines in the EU". European Medicines Agency (EMA) (Press release). 30 April 2020. Retrieved 2 June 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Ranitidine-containing medicinal products". European Medicines Agency (EMA). 30 April 2020. Retrieved 6 May 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Ranitidine". Therapeutic Goods Administration (TGA). 2 April 2020. Archived from the original on 29 August 2021. Retrieved 19 July 2020.
- ↑ "Class 2 Medicines Recall: Rosemont Pharmaceuticals Limited, Metformin Hydrochloride 500mg/5ml Oral Solution, PL 00427/0139, EL (21)A/20". GOV.UK. Retrieved 2021-08-27.
- ↑ Ogbede, J.U., Giaever, G. & Nislow, C. A genome-wide portrait of pervasive drug contaminants. Sci Rep 11, 12487 (2021). https://doi.org/10.1038/s41598-021-91792-1


