6.png.webp) | |
 | |
| Names | |
|---|---|
| IUPAC name
Hexacarbonylchromium | |
| Other names
Chromium carbonyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.579 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cr(CO)6 | |
| Molar mass | 220.057 g/mol |
| Appearance | colorless crystals |
| Density | 1.77 g/cm3, solid |
| Melting point | 90 °C (194 °F; 363 K) |
| Boiling point | 210 °C (410 °F; 483 K) (decomposes) |
| insoluble | |
| Solubility | soluble in organic solvents |
| Structure | |
| orthrhombic | |
| octahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Toxic |
| NFPA 704 (fire diamond) | |
| Flash point | 210 °C (410 °F; 483 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
150 mg/kg (oral, mouse) 230 mg/kg (oral, rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1 mg/m3[1] |
REL (Recommended) |
TWA 0.5 mg/m3[1] |
IDLH (Immediate danger) |
250 mg/m3[1] |
| Safety data sheet (SDS) | Oxford MSDS |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chromium hexacarbonyl (IUPAC name: hexacarbonylchromium) is a chromium(0) organometallic compound with the formula Cr(CO)6. It is a homoleptic complex, which means that all the ligands are identical. It is a colorless crystalline air-stable solid, with a high vapor pressure.
Preparation
Like many metal carbonyls, Cr(CO)6 is generally prepared by "reductive carbonylation", which involves reduction of a metal halide with under an atmosphere of carbon monoxide. As described in a 2023 survey of methods "most cost-effective routes for the synthesis of group 6 hexacarbonyls are based on the reduction of the metal chlorides (CrCl3, MoCl5 or WCl6) with magnesium, zinc or aluminium powders... under CO pressures".[3]
Early work on methods included controbutions from luminaries such as Walter Hieber, his student Ernst Otto Fischer, and Giulio Natta. Using specially produced chromium metal will react with CO gas to give Cr(CO)6 directly, although the method is not used commercially.
Electronic structure and bonding
In chromium hexacarbonyl, the oxidation state for chromium is assigned as zero, because Cr-C bonding electrons come from the C atom and are still assigned to C in the hypothetical ionic bond which determines the oxidation states. The formula conforms to the 18-electron rule and the complex adopts octahedral geometry with six carbonyl ligands.
The bonding between d6 chromium metal and neutral carbonyl ligands is described by the Dewar-Chatt-Duncanson model.It involves donation of electrons in HOMO of CO to empty d orbitals of the Cr metals while back-bonding from other d orbitals to the pi* orbital of the ligands reinforces the interactions synergistically.
6.png.webp)
The crystallographic studies on this compound have discovered the Cr–C and C–O distances of 1.916 and 1.171 Å, respectively.[4][5][6] On one hand, there has been continuous efforts to calculate the electronic structures (including HOMO and LUMO) as well as its molecular geometry on the chromium hexacarbonyl compound with various approaches.[7][8][9] According to one of the most recent studies,[10] the ground state configuration of Cr(CO)6 turns out (2t2g)6(9 t1u)0(2t2u)0.
Reactions and applications
Photochemical reactions
Pentacarbonyl derivatives
When heated or UV-irradiated in tetrahydrofuran (THF) solution, Cr(CO)6 converts to Cr(CO)5(THF) with loss of one CO ligand. The THF ligand is readily displaced. Often the THF complex is generated and used in situ.[11][12]
UV-irradiation of frozen solutions of chromium hexacarbonyl affords a variety of labile adducts, including labile but complexes with some noble gases.[13]
Photodimerization of norbornadiene
Norbornadiene was dimerized photochemically in the presence of Cr(CO)6, similarly to other metal complexes like Fe(CO)5, Ni(CO)4, and Co(CO)3(NO).[14]
Arene derivatives
Heating a solution of Cr(CO)6 in an aromatic solvent results in replacement of three CO ligands. The reactions are especially favorable for electron-rich arenes:
- Cr(CO)6 + C6H5R → Cr(CO)3(C6H5R) + 3 CO
The products are "piano stool complexes". These species are typically yellow solids. One example is (benzene)chromium tricarbonyl.
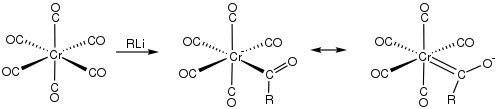
Fischer carbenes
Alkyl and aryl organolithium reagents (RLi) add to Cr(CO)6 to give anionic acyl complexes.[15] These anionic species in turn react with alkylating agents such as trimethyloxonium tetrafluoroborate [(CH3)3O]+[BF4]− to form (R−)(CH3O−)C=Cr(CO)5, where R stands for alkyl, to give Fischer carbene complexes:[16]
Cyclopentadienyl derivatives
Treatment of chromium hexacarbonyl with sodium cyclopentadienide gives Na+[Cr(CO)3(C5H5)]−. Oxidation of this salt affords cyclopentadienylchromium tricarbonyl dimer ((C5H5)2Cr2(CO)6). This complex is distinctive because it exists in measurable equilibrium with the monometallic Cr(I) radical •Cr(CO)3(C5H5).
Ligand-transfer reactions
A unique double ligand-transfer reaction was reported with using chromium trichloride and chromium hexacarbonyl.[17] In reactions, potassium perrhenate (KReO4) is reduced and carbonylated by the chromium reagents and undergoes [C5H5]− ligand-transfer to afford •Rh(CO)3(C5H5) complex derivatives.

Safety
In common with many of the other homoleptic metal carbonyls (e.g. nickel carbonyl and iron carbonyl), chromium hexacarbonyl is toxic and thought to be carcinogenic. Its vapor pressure is relatively high for a metal complex, 1 mmHg (130 Pa) at 36 °C.[18]
Historic literature
- Job, André (1927). Le chrome carbonyle et sa préparation par les organomagnésiens. impr. P. Dupont. OCLC 494793545.
- Hieber, W.; Mühlbauer, F. (1935-01-28). "Über Metallcarbonyle. XII. Reaktionen und Derivate der Hexacarbonyle des Chroms und Molybdäns". Zeitschrift für anorganische und allgemeine Chemie. 221 (4): 337–348. doi:10.1002/zaac.19352210404.
- Owen, Benton B.; English, James; Cassidy, Harold G.; Dundon, Clarissa Vanderbilt (July 1947). "The Synthesis of Chromium Hexacarbonyl 1,2". Journal of the American Chemical Society. 69 (7): 1723–1725. doi:10.1021/ja01199a044. ISSN 0002-7863.
- Natta, G.; Ercoli, R.; Calderazzo, F.; Rabizzoni, A. (July 1957). "A New Synthesis of the Chromium Hexacarbonyl". Journal of the American Chemical Society. 79 (13): 3611–3612. doi:10.1021/ja01570a092. ISSN 0002-7863.
- Rieke, Reuben D.; Öfele, Karl; Fischer, E. O. (1974-08-13). "Activated metals: VIII. Preparation of chromium hexacarbonyl from chromium metal". Journal of Organometallic Chemistry. 76 (1): C19–C21. doi:10.1016/S0022-328X(00)90328-0. ISSN 0022-328X.
References
- 1 2 3 NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Even, J.; Yakushev, A.; Dullmann, C. E.; Haba, H.; Asai, M.; Sato, T. K.; Brand, H.; Di Nitto, A.; Eichler, R.; Fan, F. L.; Hartmann, W.; Huang, M.; Jager, E.; Kaji, D.; Kanaya, J.; Kaneya, Y.; Khuyagbaatar, J.; Kindler, B.; Kratz, J. V.; Krier, J.; Kudou, Y.; Kurz, N.; Lommel, B.; Miyashita, S.; Morimoto, K.; Morita, K.; Murakami, M.; Nagame, Y.; Nitsche, H.; et al. (2014). "Synthesis and detection of a seaborgium carbonyl complex". Science. 345 (6203): 1491–3. Bibcode:2014Sci...345.1491E. doi:10.1126/science.1255720. PMID 25237098. S2CID 206558746. (subscription required)
- ↑ Bruno, Sofia M.; Valente, Anabela A.; Gonçalves, Isabel S.; Pillinger, Martyn (2023). "Group 6 Carbonyl Complexes of N,O,P-Ligands as Precursors of High-Valent Metal-Oxo Catalysts for Olefin Epoxidation". Coordination Chemistry Reviews. 478: 214983. doi:10.1016/j.ccr.2022.214983. S2CID 255329673.
- ↑ Jost, A.; Rees, B.; Yelon, W. B. (1975-11-01). "Electronic structure of chromium hexacarbonyl at 78 K. I. Neutron diffraction study". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 31 (11): 2649–2658. doi:10.1107/s0567740875008394. ISSN 0567-7408.
- ↑ Whitaker, A.; Jeffery, J. W. (1967-12-10). "The crystal structure of chromium hexacarbonyl". Acta Crystallographica. 23 (6): 977–984. doi:10.1107/S0365110X67004153.
- ↑ Kunze, Kathryn L.; Davidson, Ernest R. (March 1992). "Energetics and electronic structure of chromium hexacarbonyl". The Journal of Physical Chemistry. 96 (5): 2129–2141. doi:10.1021/j100184a022. ISSN 0022-3654.
- ↑ Johnson, Jeffrey B.; Klemperer, W. G. (October 1977). "A molecular orbital analysis of electronic structure and bonding in chromium hexacarbonyl". Journal of the American Chemical Society. 99 (22): 7132–7137. doi:10.1021/ja00464a006. ISSN 0002-7863.
- ↑ Rees, Bernard; Mitschler, Andre (December 1976). "Electronic structure of chromium hexacarbonyl at liquid nitrogen temperature. 2. Experimental study (x-ray and neutron diffraction) of .sigma. and .pi. bonding". Journal of the American Chemical Society. 98 (25): 7918–7924. doi:10.1021/ja00441a005. ISSN 0002-7863.
- ↑ Schreiner, A. F.; Brown, Theodore L. (June 1968). "A semiempirical molecular orbital model for Cr(CO)6, Fe(CO)5, and Ni(CO)4". Journal of the American Chemical Society. 90 (13): 3366–3374. doi:10.1021/ja01015a013. ISSN 0002-7863.
- ↑ Rosa, Angela; Baerends, Evert Jan; van Gisbergen, Stan J. A.; van Lenthe, Erik; Groeneveld, Jeroen A.; Snijders, Jaap G. (1999-11-01). "Electronic Spectra of M(CO) 6 (M = Cr, Mo, W) Revisited by a Relativistic TDDFT Approach". Journal of the American Chemical Society. 121 (44): 10356–10365. doi:10.1021/ja990747t. ISSN 0002-7863.
- ↑ Costamagna, J. A.; Granifo, J. (1985). "(Substituted Thiourea)Pentacarbonylchromium(0) Complexes". Inorganic Syntheses. Inorganic Syntheses. Vol. 23. pp. 1–4. doi:10.1002/9780470132548.ch1. ISBN 9780470132548.
- ↑ Simon, John D.; Xie, Xiaoliang (December 1986). "Photodissociation of chromium hexacarbonyl in solution: direct observation of the formation of pentacarbonyl(methanol)chromium". The Journal of Physical Chemistry. 90 (26): 6751–6753. doi:10.1021/j100284a005. ISSN 0022-3654.
- ↑ Perutz, Robin N.; Turner, James J. (1975). "Photochemistry of the Group 6 Hexacarbonyls in Low-Temperature Matrixes. III. Interaction of the Pentacarbonyls with Noble Gases and Other Matrixes". Journal of the American Chemical Society. 97 (17): 4791–800. doi:10.1021/ja00850a001.
- ↑ Jennings, Wyn (1970). "Photodimerization of norbornadiene using chromium hexacarbonyl". Journal of the American Chemical Society. 92 (10): 3199–3200. doi:10.1021/ja00713a055.
- ↑ Elschenbroich, C. (2006). Organometallics. Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ↑ Herndon, James W. (2001). "Pentacarbonyl(methoxyphenylcarbene)chromium(0)". e-EROS Encyclopedia of Reagents for Organic Synthesis.
- ↑ Katzenellenbogen, John (1998). "Preparation of Cyclopentadienyltricarbonylrhenium Complexes Using a Double Ligand-Transfer Reaction". Organometallics. 17 (10): 2009–2017. doi:10.1021/om971018u.
- ↑ Patnaik, Pradyot (2003). "Chromium hexacarbonyl". Handbook of Inorganic Chemicals. McGraw-Hill Professional. pp. 222–223. ISBN 978-0-07-049439-8.