 | |
 | |
| Names | |
|---|---|
| IUPAC names
Peroxysulfuric acid Sulfuroperoxoic acid[1] | |
| Systematic IUPAC name | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.879 |
| EC Number |
|
| 101039 | |
PubChem CID |
|
| UNII | |
| UN number | 1483 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H 2SO 5 | |
| Molar mass | 114.078 g mol−1 |
| Appearance | White crystals |
| Density | 2.239 g cm−3 |
| Melting point | 45 °C |
| Conjugate base | Peroxomonosulfate |
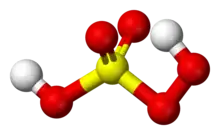
| Structure | |
| Tetrahedral at S | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
strong oxidizer |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Peroxymonosulfuric acid, H
2SO
5, is also known as persulfuric acid, peroxysulfuric acid, or Caro's acid. In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. It is one of the strongest oxidants known (E0 = +2.51 V) and is highly explosive.
H
2SO
5 is sometimes confused with H
2S
2O
8, known as peroxydisulfuric acid. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO–S(O)2–O–O–S(O)2–OH.
History
H
2SO
5 was first described in 1898 by the German chemist Heinrich Caro, after whom it is named.[3]
Synthesis and production
The laboratory scale preparation of Caro's acid involves the combination of chlorosulfuric acid and hydrogen peroxide:
- H
2O
2 + ClSO
2OH ⇌ H
2SO
5 + HCl [4]
- H
Published patents include more than one reaction for preparation of Caro's acid, usually as an intermediate for the production of potassium monopersulfate (PMPS), a bleaching and oxidizing agent. One patent for production of Caro's acid for this purpose gives the following reaction:
- H
2O
2 + H
2SO
4 ⇌ H
2SO
5 + H
2O [5]
- H
This is the reaction that produces the acid transiently in "piranha solution".
Uses in industry
H
2SO
5 has been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. Alkali metal salts of H
2SO
5 show promise for the delignification of wood.[6] It is also used in laboratories as a last resort in removing organic materials since H
2SO
5 can fully oxidize any organic materials.
Ammonium, sodium, and potassium salts of H
2SO
5 are used in the plastics industry as radical initiators for polymerization. They are also used as etchants, oxidative desizing agents for textile fabrics, and for decolorizing and deodorizing oils.
Potassium peroxymonosulfate, KHSO
5, is the potassium acid salt of peroxymonosulfuric acid. It is widely used as an oxidizing agent.
Hazards
Pure Caro's acid is highly explosive. Explosions have been reported at Brown University[7] and Sun Oil. As with all strong oxidizing agents, peroxysulfuric acid should be kept away from organic compounds such as ethers and ketones because of its ability to peroxidize these compounds, creating highly unstable molecules such as acetone peroxide.
See also
References
- 1 2 3 International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 139. Electronic version.
- ↑ "Peroxysulfuric acid (CHEBI:29286)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 20 November 2007. Retrieved 17 November 2011.
- ↑ Caro, H. (1898). "Zur Kenntniss der Oxydation aromatischer Amine" [[Contribution] to [our] knowledge of the oxidation of aromatic amines]. Zeitschrift für angewandte Chemie. 11 (36): 845–846. doi:10.1002/ange.18980113602.
- ↑ "Synthesis of Caro's acid". PrepChem.com. 2017-02-13. Retrieved 2018-10-12.
- ↑ A method and apparatus for producing a peroxyacid solution, retrieved 2018-10-12
- ↑ Springer, E. L.; McSweeny, J. D. (1993). "Treatment of softwood kraft pulps with peroxymonosulfate before oxygen delignification". TAPPI Journal. 76 (8): 194–199. ISSN 0734-1415. Archived from the original on 2011-09-29. Retrieved 2011-05-14.
- ↑ Edwards, J.O. (1955). "Safety". Chem. Eng. News. 33 (32): 3336. doi:10.1021/cen-v033n032.p3336.