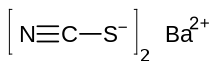 | |
| Names | |
|---|---|
| IUPAC name
Barium thiocyanate | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.016.587 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Ba(SCN)2 | |
| Molar mass | 253.49 g/mol |
| Appearance | White crystals |
| 62.63 g/100 ml (25°C) | |
| Solubility | Soluble in acetone, methanol, and ethanol |
| Hazards | |
| GHS labelling: | |
 | |
| H301, H312, H315, H319, H332, H335 | |
| P261, P280, P302+P352, P304+P340 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Barium thiocyanate is a colorless water-soluble salt that is very hygroscopic. It is highly toxic to ingestion and irritates the skin. It is also soluble in most alcohols and insoluble in simple alkanes.
Uses
Barium thiocyanate is used in dyeing textiles and is an ingredient in some photographic solutions. But because of its toxicity, it has limited uses.[3]
Preparation
Barium thiocyanate is prepared by dissolving barium metal or barium nitrate in a solution of thiocyanic acid.
References
- ↑ "Barium thiocyanate | 336879-43-7". Sigma-Aldrich. 2012-09-14. Retrieved 2021-01-20.
- ↑ "BARIUM THIOCYANATE | 2092-17-3". Chemicalbook.com. 2020-07-03. Retrieved 2021-01-20.
- ↑ "Barium thiocyanate - CAMEO". Cameo.mfa.org. 2016-04-29. Retrieved 2021-01-20.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.