 | |
 | |
| Names | |
|---|---|
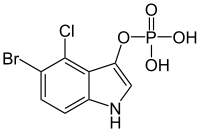
| Preferred IUPAC name
5-Bromo-4-chloro-1H-indol-3-yl dihydrogen phosphate | |
| Other names
BCIP | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6BrClNO4P | |
| Molar mass | 326.47 g·mol−1 |
| Appearance | Colorless |
| soluble in water (sodium salt) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5-Bromo-4-chloro-3-indolyl phosphate (BCIP, X-phosphate, XP) is an artificial chromogenic substrate used for the sensitive colorimetric detection of alkaline phosphatase activity. It is, for example, used in immunoblotting, in situ hybridization, and immunohistochemistry, often in combination with nitro blue tetrazolium chloride (NBT).[1][2] 5-bromo-4-chloro-3-indoxyl is oxidized by atmospheric oxygen to form the blue dye 5,5′-dibromo-4,4′-dichloro-indigo. It is also oxidized by nitroblue tetrazolium (NBT), which forms an insoluble dark blue diformazan precipitate after reduction. Alkaline phosphatase is commonly conjugated to secondary antibodies.
References
- ↑ "5-Bromo-4-chloro-3-indolyl phosphate disodium salt". Millipore Sigma.
- ↑ Alkaline phosphatase hydrolyses BCIP to 5-bromo-4-chloro-3-indoxyl<JP Horwitz J. Med. Chem., 1966, 9 (3), pp 447–447 and inorganic phosphate
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.