 | |
| Names | |
|---|---|
| Other names
Antrin; Lu texaphyrin; Lu-Tex; Lutetium texaphyrin; Lutrin; Optrin; PCI 0123 | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
| |
| Properties | |
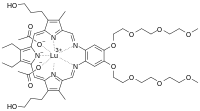
| C52H72LuN5O14 | |
| Molar mass | 1166.136 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Motexafin lutetium is a texaphyrin, marketed as Antrin by Pharmacyclics Inc.
It is a photosensitiser for use in photodynamic therapy to treat skin conditions and superficial cancers.
It has also been tested for use in photoangioplasty (photodynamic treatment of diseased arteries).[1]
It is photoactivated by 732 nm light which allows greater depth of penetration.[2]
Clinical trials
Phase II clinical trials were in progress in 1999.[3]
A phase I trial for prostate cancer reported in 2009.[4]
References
- ↑ Pharmacyclics Announces Final Phase 1 Results of Antrin Phototherapy For Coronary Artery Disease Archived 2011-07-16 at the Wayback Machine, 2002
- ↑ http://findarticles.com/p/articles/mi_qa3931/is_200909/ai_n42040200/pg_9/ Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy Photochemistry and Photobiology, Sep/Oct 2009. by O'Connor, Aisling E, Gallagher, William M, Byrne, Annette T
- ↑ Antrin Photoangioplasty Phase II Clinical Trial Patient Treated During Live Case Demonstration at TCT Archived 2012-07-13 at archive.today, 1999
- ↑ Patel, H; Mick, R; Finlay, J; Zhu, TC; Rickter, E; Cengel, KA; Malkowicz, SB; Hahn, SM; Busch, TM (2008). "Motexafin lutetium-photodynamic therapy of prostate cancer: Short and long term effects on PSA". Clinical Cancer Research. 14 (15): 4869–76. doi:10.1158/1078-0432.CCR-08-0317. PMC 2680073. PMID 18676760.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.