 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | 18% |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H21NO |
| Molar mass | 243.350 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
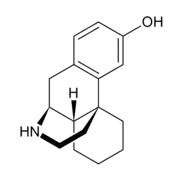
3-Hydroxymorphinan (3-HM), or morphinan-3-ol, is a psychoactive drug of the morphinan family.[1] It is the racemic counterpart to norlevorphanol.
The dextrorotatory stereoisomer of the compound is an active metabolite of dextromethorphan, dextrorphan, and 3-methoxymorphinan,[2] and similarly to them has potent neuroprotective and neurotrophic effects on LTS- and MPTP-treated dopaminergic neurons of the nigrostriatal pathway,[3][4] but notably without producing any neuropsychotoxic side effects (e.g., dissociation or hallucinations) or having any anticonvulsant actions.[5][6] It does not seem to bind to the NMDA receptor,[6] and instead, its neuroprotective properties appear result from inhibition of glutamate release via the suppression of presynaptic voltage-dependent Ca2+ entry and protein kinase C activity.[7] In any case, as such, the compound has been investigated as a potential management of Parkinson's disease medication (antiparkinsonian agent). A prodrug, GCC1290K, has been developed on account of 3-HM's poor bioavailability (18%), and a New Drug Application has been approved for it by the United States Food and Drug Administration.[6] It is currently undergoing clinical trials for the treatment of Parkinson's disease.[6] It does not have a Controlled Substances Act 1970 schedule, ACSCN, or annual aggregate manufacturing quota and may not necessarily be controlled, whilst norlevorphanol is; none of the dextrorotary derivatives of the dromoran and norlevorphanol sub-families of morphinan derivatives are controlled as they do not have opioid activity but the other racemic compounds are.[8]
3-HM's levorotatory stereoisomer, norlevorphanol, in contrast to (+)-3-HM, is an opioid analgesic.[9] It was never marketed as such however, probably due to a combination of the facts that norlevorphanol has low bioavailability and that its potency is diminished compared to its N-methylated analogue levorphanol.[10]
-3-Hydroxymorphinan.png.webp)
References
- ↑ Ganellin CR, Triggle DJ, Macdonald F (1997). Dictionary of pharmacological agents. CRC Press. p. 1378. ISBN 978-0-412-46630-4. Retrieved 29 November 2011.
- ↑ Jacqz-Aigrain E, Cresteil T (1992). "Cytochrome P450-dependent metabolism of dextromethorphan: fetal and adult studies". Developmental Pharmacology and Therapeutics. 18 (3–4): 161–8. doi:10.1159/000480616. PMID 1306804.
- ↑ Zhang W, Qin L, Wang T, et al. (March 2005). "3-hydroxymorphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS-induced neurotoxicity". The FASEB Journal. 19 (3): 395–7. doi:10.1096/fj.04-1586fje. PMID 15596482. S2CID 13513164.
- ↑ Zhang W, Shin EJ, Wang T, et al. (December 2006). "3-Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro". The FASEB Journal. 20 (14): 2496–511. doi:10.1096/fj.06-6006com. PMID 17142799. S2CID 23032934.
- ↑ Shin EJ, Lee PH, Kim HJ, Nabeshima T, Kim HC (January 2008). "Neuropsychotoxicity of abused drugs: potential of dextromethorphan and novel neuroprotective analogs of dextromethorphan with improved safety profiles in terms of abuse and neuroprotective effects". Journal of Pharmacological Sciences. 106 (1): 22–7. doi:10.1254/jphs.fm0070177. PMID 18198471.
- 1 2 3 4 Shin EJ, Bach JH, Lee SY, et al. (2011). "Neuropsychotoxic and neuroprotective potentials of dextromethorphan and its analogs". Journal of Pharmacological Sciences. 116 (2): 137–48. doi:10.1254/jphs.11r02cr. PMID 21606622.
- ↑ Lin TY, Lu CW, Wang SJ (July 2009). "Inhibitory effect of glutamate release from rat cerebrocortical synaptosomes by dextromethorphan and its metabolite 3-hydroxymorphinan". Neurochemistry International. 54 (8): 526–34. doi:10.1016/j.neuint.2009.02.012. PMID 19428798. S2CID 23428637.
- ↑ "Conversion Factors for Controlled Substances". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice. Archived from the original on 2016-03-02. Retrieved 2016-02-27.
- ↑ Dictionary of organic compounds. London: Chapman & Hall. 1996. ISBN 0-412-54090-8.
- ↑ Bentham Science Publishers (April 1995). Current Medicinal Chemistry. Bentham Science Publishers. p. 425. Retrieved 29 November 2011.