 | |
octane-3D-spacefill.png.webp) | |
| Names | |
|---|---|
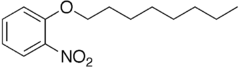
| Preferred IUPAC name
1-Nitro-2-(octyloxy)benzene | |
| Other names
1-(2-Nitrophenoxy)octane 2-Nitrophenyl octyl ether 1-Nitro-2-octoxy-benzene 2-(Octyloxy)nitrobenzene Octyl o-nitrophenyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | NPOE |
| ChemSpider | |
| ECHA InfoCard | 100.048.731 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H21NO3 | |
| Molar mass | 251.321 |
| Density | 1.04 g/mL |
| Boiling point | 197 to 198 °C (387 to 388 °F; 470 to 471 K) (11 mm Hg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1-(2-Nitrophenoxy)octane, also known as nitrophenyl octyl ether and abbreviated NPOE, is a chemical compound that is used as a matrix in fast atom bombardment mass spectrometry, liquid secondary ion mass spectrometry, and as a highly lipophilic plasticizer in polymer membranes used in ion selective electrodes.[1][2]
See also
References
- ↑ Velický, Matěj; Tam, Kin Y.; Dryfe, Robert A.W. (2014-01-07). "Mechanism of Ion Transfer in Supported Liquid Membrane Systems: Electrochemical Control over Membrane Distribution". Analytical Chemistry. 86 (1): 435–442. doi:10.1021/ac402328w. ISSN 0003-2700. PMC 3917230. PMID 24299270.
- ↑ Jorge, Miguel; Cordeiro, M. Natália D. S. (2008-02-01). "Molecular Dynamics Study of the Interface between Water and 2-Nitrophenyl Octyl Ether". The Journal of Physical Chemistry B. 112 (8): 2415–2429. doi:10.1021/jp710018q. ISSN 1520-6106. PMID 18247602.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.