| 1-aminocyclopropane-1-carboxylate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
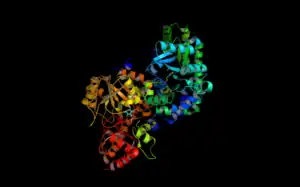
 Structure of ACC Synthase | |||||||||
| Identifiers | |||||||||
| EC no. | 4.4.1.14 | ||||||||
| CAS no. | 72506-68-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The enzyme aminocyclopropane-1-carboxylic acid synthase (ACC synthase, ACS) (EC 4.4.1.14) catalyzes the synthesis of 1-Aminocyclopropane-1-carboxylic acid (ACC), a precursor for ethylene, from S-Adenosyl methionine (AdoMet, SAM), an intermediate in the Yang cycle and activated methyl cycle and a useful molecule for methyl transfer:
- S-adenosyl-L-methionine = 1-aminocyclopropane-1-carboxylate + S-methyl-5′-thioadenosine
Like other PLP dependent enzymes, it catalyzes the reaction through a quinonoid zwitterion intermediate and uses cofactor pyridoxal phosphate (PLP, the active form of vitamin B6) for stabilization.[1][2][3]
This enzyme belongs to the family of lyases, specifically carbon-sulfur lyases. The systematic name of this enzyme class is S-adenosyl-L-methionine S-methyl-5′-thioadenosine-lyase (1-aminocyclopropane-1-carboxylate-forming). Other names in common use include 1-aminocyclopropanecarboxylate synthase, 1-aminocyclopropane-1-carboxylic acid synthase, 1-aminocyclopropane-1-carboxylate synthetase, aminocyclopropanecarboxylic acid synthase, aminocyclopropanecarboxylate synthase, ACC synthase, and S-adenosyl-L-methionine methylthioadenosine-lyase. This enzyme participates in propanoate metabolism. It employs one cofactor, pyridoxal phosphate.
Enzyme mechanism


The reaction catalyzed by 1-aminocyclopropane-1-carboxylic acid synthase (ACS) is the committed and rate-limiting step in the biosynthesis of ethylene [20], a gaseous plant hormone that is responsible for the initiation of fruit ripening, shoot and root growth and differentiation, leaf and fruit abscission, flower opening, and flower and leaf senescence. (source) It is a pyridoxal phosphate (PLP) dependent gamma-elimination (?). In the gamma elimination, PLP acts as a sink twice (absorbing electrons from two deprotonations).[4][5]
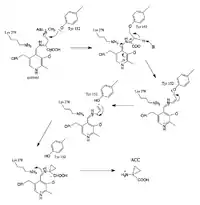
Proposed steps of the reaction mechanism:
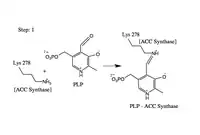
- Formation of the ACS-PLP Schiff Base
- Imine Exchange
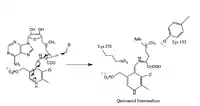
- Formation of the Quinonoid Intermediate
- Tyrosine and PLP stabilized 3C-Ring formation
- Formation of the ACS-PLP Schiff Base
The aldehyde of coenzyme PLP reacts to form an imine (Schiff base) linkage with the catalytic domain lysine (278) residue of ACS.
- Imine exchange
An imine exchange occurs, and the amine nitrogen of the substrate, S-Adenosyl methionine, replaces Lys (278) in the imine linkage. (Stabilized by H bonding).
- Formation of the Quinonoid Intermediate
PLP acts as an 'electron sink' absorbing delocalized electron density during the reaction intermediates (countering the excess electron density on the deprotonated a-carbon). PLP facilitates the enzyme activity, increasing the acidity of the alpha carbon by stabilizing the conjugate base. The PLP-stabilized carbanion intermediate formed is the quinonoid intermediate.
- Tyrosine and PLP stabilized 3C-Ring formation
PLP and Tyrosine stabilize negative charges during deprotonation. Tyrosine attacks the sulfur bound carbon, allowing S(CH3)(Ado) to leave, and during ring formation, Tyrosine leaves.
Regulation
ACC synthase reaches optimal activity in conditions of pH 8.5 and with Km = 20 um relative to its substrate, SAM.
ACC Synthase and ethylene biosynthesis are regulated by a whole host of stimuli. Stresses such as wounding, noxious chemicals, auxin, flooding, and indole-3-acetic acid (IAA) promote ethylene synthesis, creating a positive feedback cycle with ACC synthase, up-regulating its activity.
However, it is also inhibited by a number of compounds as well. S-Adenosylethionine can bind as a substrate for ACC synthase (with higher affinity than SAM) and therefore inhibit any reaction with SAM. ACC Synthase is also competitively inhibited by aminoethoxyvinylglycine (AVG) and aminooxyacetic acid (AOA), inhibitors to many pyridoxal phosphate-mediated enzymic reactions. They are natural toxins that cause slow binding inhibition by interfering with the coenzyme pyridoxal phosphate. ACC synthase activity is also inhibited by intermediates of the activated methyl cycle and the methionine-recycling pathway: 5′-methylthioadenosine, α-keto-γ-methylthiobutyric acid, and S-adenosylhomocysteine.[8][9][10]
Structure
ACC Synthase is 450-516 amino acid long sequence depending on the species of plant from which it is extracted. Though it is comparable in the species in which it is found, its COOH terminal domain is more variable, leading to differences such as oligomerization. The COOH terminal domain is responsible for oligomerization. In most ACC Synthase producing cells, ACC Synthase exists as a dimer. However, in some we find a monomer ("which is more active and efficient [than its dimer counterpart").[11]
The structure of ACS has been largely determined via X-ray crystallography.[12] Conservation of the residues in ACS's catalytic domain and sequence homology suggest that ACS catalyzes the synthesis of ACC in a similar fashion as other enzymes that require PLP as a cofactor. However, unlike many other PLP-dependent enzymes, Lys (278) is not the only residue that interacts with the substrate. The proximity of the electronegative oxygen from Tyr (152) to the C-γ-S bond suggests a crucial role in the formation of ACC.[13] X-ray crystallography with aminoethoxyvinylglycine (AVG) a competitive inhibitor confirmed Tyrosine's role in the γ elimination.[14]
As of late 2007, 6 structures have been solved for this class of enzymes, with PDB accession codes 1B8G, 1IAX, 1IAY, 1M4N, 1M7Y, and 1YNU.

Catalytic domain
The main functional groups in the catalytic domains are the Nitrogen from the Lys 278 residue and the Oxygen from the Tyrosine 152 residue.
Biological function and applications
ACC Synthase is the key, rate limiting step in ethylene synthesis. Because the up-regulation of ACC-Synthase is what induces fruit ripening and often spoilage there is more research being done on the regulatory mechanisms and biosynthetic pathways of ethylene to avoid spoilage.[15][16]
Notes
- ↑ Zhang Z, Ren JS, Clifton IJ, Schofield CJ (October 2004). "Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase—the ethylene-forming enzyme". Chem. Biol. 11 (10): 1383–94. doi:10.1016/j.chembiol.2004.08.012. PMID 15489165.
- ↑ Capitani G, Hohenester E, Feng L, Storici P, Kirsch JF, Jansonius JN (December 1999). "Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene". J. Mol. Biol. 294 (3): 745–56. doi:10.1006/jmbi.1999.3255. PMID 10610793.
- ↑ Huai Q, Xia Y, Chen Y, Callahan B, Li N, Ke H (October 2001). "Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5'-phosphate provide new insight into catalytic mechanisms". J. Biol. Chem. 276 (41): 38210–6. doi:10.1074/jbc.M103840200. PMID 11431475.
- ↑ Li JF, Qu LH, Li N (August 2005). "Tyr152 plays a central role in the catalysis of 1-aminocyclopropane-1-carboxylate synthase". J. Exp. Bot. 56 (418): 2203–10. doi:10.1093/jxb/eri220. PMID 15983009.
- ↑ Capitani G, McCarthy DL, Gut H, Grütter MG, Kirsch JF (December 2002). "Apple 1-aminocyclopropane-1-carboxylate synthase in complex with the inhibitor L-aminoethoxyvinylglycine. Evidence for a ketimine intermediate". J. Biol. Chem. 277 (51): 49735–42. doi:10.1074/jbc.M208427200. PMID 12228256. S2CID 1019316.
- ↑ Huai Q, Xia Y, Chen Y, Callahan B, Li N, Ke H (October 2001). "Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5'-phosphate provide new insight into catalytic mechanisms". J. Biol. Chem. 276 (41): 38210–6. doi:10.1074/jbc.M103840200. PMID 11431475.
- ↑ Wang KL, Li H, Ecker JR (2002). "Ethylene biosynthesis and signaling networks". Plant Cell. 14 Suppl: S131–51. doi:10.1105/tpc.001768. PMC 151252. PMID 12045274.
- ↑ Yip WK, Moore T, Yang SF (March 1992). "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. Bibcode:1992PNAS...89.2475Y. doi:10.1073/pnas.89.6.2475. PMC 48681. PMID 1549612.
- ↑ Acaster MA, Kende H (May 1983). "Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase". Plant Physiol. 72 (1): 139–45. doi:10.1104/pp.72.1.139. PMC 1066183. PMID 16662947.
- ↑ Lewis DR, Negi S, Sukumar P, Muday GK (August 2011). "Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers". Development. 138 (16): 3485–95. doi:10.1242/dev.065102. PMID 21771812. S2CID 7211767.
- ↑ Kathiresan A, Nagarathna KC, Moloney MM, Reid DM, Chinnappa CC (January 1998). "Differential regulation of 1-aminocyclopropane-1-carboxylate synthase gene family and its role in phenotypic plasticity in Stellaria longipes". Plant Mol. Biol. 36 (2): 265–74. doi:10.1023/A:1005994118535. PMID 9484438. S2CID 9161137.
- ↑ Yip WK, Moore T, Yang SF (March 1992). "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. Bibcode:1992PNAS...89.2475Y. doi:10.1073/pnas.89.6.2475. PMC 48681. PMID 1549612.
- ↑ Yip WK, Moore T, Yang SF (March 1992). "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. Bibcode:1992PNAS...89.2475Y. doi:10.1073/pnas.89.6.2475. PMC 48681. PMID 1549612.
- ↑ Yip WK, Moore T, Yang SF (March 1992). "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. Bibcode:1992PNAS...89.2475Y. doi:10.1073/pnas.89.6.2475. PMC 48681. PMID 1549612.
- ↑ Acaster MA, Kende H (May 1983). "Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase". Plant Physiol. 72 (1): 139–45. doi:10.1104/pp.72.1.139. PMC 1066183. PMID 16662947.
- ↑ Nakatsuka A, Murachi S, Okunishi H, et al. (December 1998). "Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening". Plant Physiol. 118 (4): 1295–305. doi:10.1104/pp.118.4.1295. PMC 34745. PMID 9847103.
References
- Lin, E. C. C.; Kistler, W. S.; Zwaig, N. (June 1970). "Glycerol Kinase, the Pacemaker for the Dissimilation of Glycerol in Escherichia coli". Journal of Bacteriology. 102 (3): 753–759. doi:10.1128/JB.102.3.753-759.1970. PMC 247623. PMID 4914079.
- Nakatsuka, Akira; Murachi, Shiho; Okunishi, Hironori; Shiomi, Shinjiro; Nakano, Ryohei; Kubo, Yasutaka; Inaba, Akitsugu (1998). "Differential Expression and Internal Feedback Regulation of 1-Aminocyclopropane-1-Carboxylate Synthase, 1-Aminocyclopropane-1-Carboxylate Oxidase, and Ethylene Receptor Genes in Tomato Fruit during Development and Ripening". Plant Physiology. 118 (4): 1295–1305. doi:10.1104/pp.118.4.1295. PMC 34745. PMID 9847103.
- Wang, H.; Mei, W.; Qin, Y.; Zhu, Y. (2011). "1-Aminocyclopropane-1-carboxylic acid synthase 2 is phosphorylated by calcium-dependent protein kinase 1 during cotton fiber elongation". Acta Biochimica et Biophysica Sinica. 43 (8): 654–661. doi:10.1093/abbs/gmr056. PMID 21742672.
- Wang, Long-Chi; Hsu, Jen-Hung; Lin, Lee-Chung (2010-10-22). "Identification of Novel Inhibitors of 1-Aminocyclopropane-1-carboxylic Acid Synthase by Chemical Screening in Arabidopsis thaliana". Journal of Biological Chemistry. 285 (43): 33445–33456. doi:10.1074/jbc.M110.132498. PMC 2963424. PMID 20682786.
- "Ethylene Biosynthesis and Signaling Networks". Archived from the original on 2012-03-07.
- Guido Capitani; Markus Tschopp; Andrew C. Eliot; Jack F. Kirsch; Markus G. Grütter. "Structure of ACC synthase inactivated by the mechanism-based inhibitor L-vinylglycine" (PDF). www.bioc.uzh.ch. Retrieved 2012-03-15.
- "jxb.oxfordjournals.org" (PDF). Archived from the original (PDF) on 2018-10-30.
- Li, Jian-Feng; Qu, Liang-Hu; Li, Ning (2005). "jxb.oxfordjournals.org". Journal of Experimental Botany. 56 (418): 2203–2210. doi:10.1093/jxb/eri220. PMID 15983009.
- Koga Y, Katsumi R, You DJ, Matsumura H, Takano K, Kanaya S (May 2008). "Crystal structure of highly thermostable glycerol kinase from a hyperthermophilic archaeon in a dimeric form". FEBS J. 275 (10): 2632–43. doi:10.1111/j.1742-4658.2008.06410.x. PMID 18422647. S2CID 205878773.
- Nakatsuka A, Murachi S, Okunishi H, et al. (December 1998). "Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening". Plant Physiol. 118 (4): 1295–305. doi:10.1104/pp.118.4.1295. PMC 34745. PMID 9847103.
- Wang H, Mei W, Qin Y, Zhu Y (August 2011). "1-Aminocyclopropane-1-carboxylic acid synthase 2 is phosphorylated by calcium-dependent protein kinase 1 during cotton fiber elongation". Acta Biochim. Biophys. Sin. (Shanghai). 43 (8): 654–61. doi:10.1093/abbs/gmr056. PMID 21742672.
- Capitani G, Tschopp M, Eliot AC, Kirsch JF, Grütter MG (April 2005). "Structure of ACC synthase inactivated by the mechanism-based inhibitor L-vinylglycine". FEBS Lett. 579 (11): 2458–62. doi:10.1016/j.febslet.2005.03.048. PMID 15848188. S2CID 24574380.
- Li JF, Qu LH, Li N (August 2005). "Tyr152 plays a central role in the catalysis of 1-aminocyclopropane-1-carboxylate synthase". J. Exp. Bot. 56 (418): 2203–10. doi:10.1093/jxb/eri220. PMID 15983009.
- Capitani G, McCarthy DL, Gut H, Grütter MG, Kirsch JF (December 2002). "Apple 1-aminocyclopropane-1-carboxylate synthase in complex with the inhibitor L-aminoethoxyvinylglycine. Evidence for a ketimine intermediate". J. Biol. Chem. 277 (51): 49735–42. doi:10.1074/jbc.M208427200. PMID 12228256. S2CID 1019316.
- Huai Q, Xia Y, Chen Y, Callahan B, Li N, Ke H (October 2001). "Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5'-phosphate provide new insight into catalytic mechanisms". J. Biol. Chem. 276 (41): 38210–6. doi:10.1074/jbc.M103840200. PMID 11431475.
- Barry CS, Llop-Tous MI, Grierson D (July 2000). "The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato". Plant Physiol. 123 (3): 979–86. doi:10.1104/pp.123.3.979. PMC 59060. PMID 10889246.
- Zhang Z, Ren JS, Clifton IJ, Schofield CJ (October 2004). "Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase--the ethylene-forming enzyme". Chem. Biol. 11 (10): 1383–94. doi:10.1016/j.chembiol.2004.08.012. PMID 15489165.
- Capitani G, Hohenester E, Feng L, Storici P, Kirsch JF, Jansonius JN (December 1999). "Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene". J. Mol. Biol. 294 (3): 745–56. doi:10.1006/jmbi.1999.3255. PMID 10610793.
- Holland RR (August 1975). "Decision tables. Their use for the presentation of clinical algorithms". JAMA. 233 (5): 455–7. doi:10.1001/jama.1975.03260050061028. PMID 1080212.
- Kathiresan A, Nagarathna KC, Moloney MM, Reid DM, Chinnappa CC (January 1998). "Differential regulation of 1-aminocyclopropane-1-carboxylate synthase gene family and its role in phenotypic plasticity in Stellaria longipes". Plant Mol. Biol. 36 (2): 265–74. doi:10.1023/A:1005994118535. PMID 9484438. S2CID 9161137.
- Yip WK, Moore T, Yang SF (March 1992). "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. Bibcode:1992PNAS...89.2475Y. doi:10.1073/pnas.89.6.2475. PMC 48681. PMID 1549612.
- Acaster MA, Kende H (May 1983). "Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase". Plant Physiol. 72 (1): 139–45. doi:10.1104/pp.72.1.139. PMC 1066183. PMID 16662947.
- Lewis DR, Negi S, Sukumar P, Muday GK (August 2011). "Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers". Development. 138 (16): 3485–95. doi:10.1242/dev.065102. PMID 21771812. S2CID 7211767.
- Clausen T, Huber R, Messerschmidt A, Pohlenz HD, Laber B (October 1997). "Slow-binding inhibition of Escherichia coli cystathionine beta-lyase by L-aminoethoxyvinylglycine: a kinetic and X-ray study". Biochemistry. 36 (41): 12633–43. doi:10.1021/bi970630m. PMID 9376370.
- Wang KL, Li H, Ecker JR (2002). "Ethylene biosynthesis and signaling networks". Plant Cell. 14 Suppl: S131–51. doi:10.1105/tpc.001768. PMC 151252. PMID 12045274.
- Boller T, Herner RC, Kende H (1979). "Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid". Planta. 145 (3): 293–303. doi:10.1007/BF00454455. PMID 24317737. S2CID 27464828.
- Yu YB, Adams DO, Yang SF (1979). "1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis". Arch. Biochem. Biophys. 198 (1): 280–6. doi:10.1016/0003-9861(79)90420-X. PMID 507845.