 | |
| Names | |
|---|---|
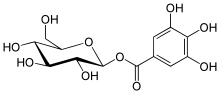
| IUPAC name
[(2S,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl]3,4,5-trihydroxybenzoate | |
| Other names
β-Glucogallin 1-Galloylglucose 1-Galloyl-β-glucose 1-O-Galloyl-β-D-glucose beta-Glucogallin Monogalloyl glucose | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.242.331 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H16O10 | |
| Molar mass | 332.261 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Glucogallin is chemical compound formed from gallic acid and β-D-glucose. It can be found in oaks species like the North American white oak (Quercus alba), European red oak (Quercus robur) [1] and Amla fruit (Phyllanthus emblica).[2]
It is formed by a gallate 1-beta-glucosyltransferase (UDP-glucose: gallate glucosyltransferase), an enzyme performing the esterification of two substrates, UDP-glucose and gallate to yield two products, UDP and glucogallin. This enzyme can be found in oak leaf preparations.[3]
This the first step in the biosynthesis of gallotannins. The molecule is then used by enzymes in the gallotannins synthetics pathway like beta-glucogallin O-galloyltransferase or beta-glucogallin-tetrakisgalloylglucose O-galloyltransferase.
β-Glucogallin is aldose reductase inhibitor.
Half-life of β-Glucogallin in human body seems to be unknown.
References
- ↑ Mämmelä, Pirjo; Savolainen, Heikki; Lindroos, Lasse; Kangas, Juhani; Vartiainen, Terttu (2000). "Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry". Journal of Chromatography A. 891 (1): 75–83. doi:10.1016/S0021-9673(00)00624-5. PMID 10999626.
- ↑ Puppala, M; Ponder, J; Suryanarayana, P; Reddy, GB; Petrash, JM; LaBarbera, DV (2012). "The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis". PLOS ONE. 7 (4): e31399. Bibcode:2012PLoSO...731399P. doi:10.1371/journal.pone.0031399. PMC 3317655. PMID 22485126.
- ↑ Gross, G.G. (1982). "Synthesis of β-glucogallin from UDP-glucose and gallic acid by an enzyme preparation from oak leaves". FEBS Letters. 148: 67–70. doi:10.1016/0014-5793(82)81244-1. S2CID 86402007.