 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylethane-1,2-diamine | |
| Other names
N,N-Dimethyl-1,2-ethanediamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.220 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H12N2 | |
| Molar mass | 88.154 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 107 °C (225 °F; 380 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
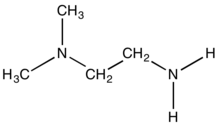
1,1-Dimethylethylenediamine is the organic compound with the formula (CH3)2N)CH2CH2NH2. It is a colorless liquid with a fishy odor. It features one primary amine and a tertiary amine. It is used to prepare a chelating diamine-containing ligands for the preparation of metal catalysts.[1][2] It is a precursor to the drug chloropyramine.
See also
References
- ↑ Chen, Hsuan-Ying; Tang, Hui-Yi; Lin, Chu-Chieh "Ring-Opening Polymerization of Lactides Initiated by Zinc Alkoxides Derived from NNO-Tridentate Ligands" Macromolecules 2006, volume 39, pp. 3745-3752. doi:10.1021/ma060471r
- ↑ Tshuva, Edit Y.; Goldberg, Israel; Kol, Moshe; Goldschmidt, Zeev "Zirconium Complexes of Amine-Bis(phenolate) Ligands as Catalysts for 1-Hexene Polymerization: Peripheral Structural Parameters Strongly Affect Reactivity" Organometallics 2001, volume 20, pp. 3017-3028. doi:10.1021/om0101285
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.